Anaspaz
Generic name:hyoscyamine sulfate
Dosage form: tablet, orally disintegrating
Drug class:Anticholinergics / antispasmodics
Medically reviewed by Drugs.com. Last updated on Sep 21, 2021.
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
l-hyoscyamine sulfate
0.125 mg tablets
Rx only
On This Page
Anaspaz Description
Each Anaspaz tablet contains l-hyoscyamine sulfate 0.125mg. Anaspaz may be taken orally (swallowed or chewed) or sublingually. Anaspaz tablets are compressed, light yellow, and scored with the Ascher logo on one side and 225/295 on the other. Inactive ingredients: FD&C yellow #6, FD&C yellow #10, lactose monohydrate NF, magnesium stearate NF, mannitol USP, starch 1500 NF, stearic acid NF purified powder.
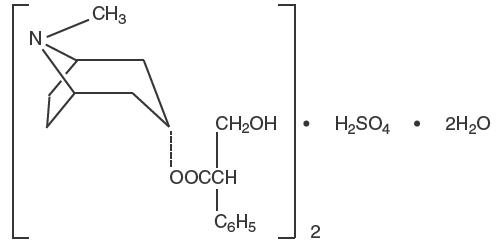
Anaspaz is chemically pure l-hyoscyamine sulfate, one of the principal anticholinergic/antispasmodic components of belladonna alkaloids. Chemically, it is benzeneacetic acid, α-(hydroxymethyl)-, 8-methyl-8-azabicyclo[3.2.1.]oct-3-yl ester, [3(S)-endo]-, sulfate (2:1), dihydrate with the chemical formula (C 17H 23NO 3)•2H 2SO 4•2H 2O.
Anaspaz - Clinical Pharmacology
Anaspaz inhibits specifically the actions of acetylcholine on structures innervated by postganglionic cholinergic nerves and on smooth muscles that respond to acetylcholine but lack cholinergic innervation. These peripheral cholinergic receptors are present in the autonomic effector cells of smooth muscle, cardiac muscle, the sino-atrial node, the atrioventricular node and the exocrine glands. At therapeutic doses, it is completely devoid of any action on autonomic ganglia. Anaspaz inhibits gastrointestinal propulsive motility and decreases gastric acid secretion. Anaspaz also controls excessive pharyngeal, tracheal and bronchial secretions. Anaspaz is absorbed totally and completely by sublingual administration as well as oral administr.



