Naphcon Forte
Generic name:naphazoline hydrochloride
Dosage form: ophthalmic solution
Drug class:Ophthalmic antihistamines and decongestants
Medically reviewed by Drugs.com. Last updated on Mar 22, 2022.
On This Page
The Naphcon Forte brand name has been discontinued in the U.S. If generic versions of this product have been approved by the FDA, there may be generic equivalents available.
DESCRIPTION
NAPHCON® FORTE (Naphazoline Hydrochloride Ophthalmic Solution USP), 0.1% is a sterile preparation. Naphazoline HCl, an ocular vasoconstrictor, is an imidazoline derivative sympathomimetic amine. It occurs as a white, odorless crystalline powder having a bitter taste and is freely soluble in water and in alcohol. The active ingredient is represented by the structural formula:
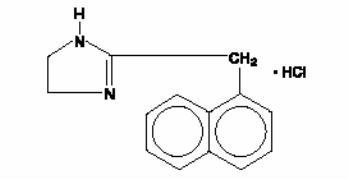
C14H14N2HCl
MW=246.74
Chemical name:
2-(1-naphthylmethyl)-2-imidazoline monohydrochloride
Each mL contains: Active: Naphazoline Hydrochloride 0.1%. Preservative: Benzalkonium Chloride 0.01%. Inactives: Boric Acid, Sodium Chloride, Potassium Chloride, Edetate Disodium, Sodium Carbonate and/or Hydrochloric Acid (to adjust pH), Purified Water. DM-00
The solution has a pH of 5.5 to 7.0.
CLINICAL PHARMACOLOGY
Naphazoline constricts the vascular system of the conjunctiva. It is presumed that this effect is due to direct stimulation action of the drug upon the alpha adrenergic receptors in the arterioles of the conjunctiva resulting in decreased conjunctival congestion. Naphazoline belongs to the imidazoline class of sympathomimetics.
INDICATIONS AND USAGE
NAPHCON® FORTE (Naphazoline Hydrochloride Ophthalmic Solution USP), 0.1% is indicated for use as a topical ocular vasoconstrictor.
CONTRAINDICATIONS
Contraindicated in the presence of an anatomically narrow angle or in narrow angle glaucoma or in persons who have shown hypersensitivity to any component of this preparation.
WARNINGS
NOT FOR INJECTION – FOR OPHTHALMIC USE ONLY. Patients under therapy...



