Anesthesia S/I-50
Generic name: propofol
Dosage form: kit
On This Page
DIPRIVAN®
FOR INTRAVENOUS ADMINISTRATION
Rx Only
Strict aseptic technique must always be maintained during handling. DIPRIVAN Injectable Emulsion is a single access parenteral product (single patient infusion vial) which contains 0.005% disodium edetate (EDTA) to inhibit the rate of growth of microorganisms, for up to 12 hours, in the event of accidental extrinsic contamination. However, DIPRIVAN Injectable Emulsion can still support the growth of microorganisms, as it is not an antimicrobially preserved product under USP standards. Do not use if contamination is suspected. Discard unused drug product as directed within the required time limits. There have been reports in which failure to use aseptic technique when handling DIPRIVAN Injectable Emulsion was associated with microbial contamination of the product and with fever, infection/sepsis, other lifethreatening illness, and/or death.
There have been reports, in the literature and other public sources, of the transmission of bloodborne pathogens (such as Hepatitis B, Hepatitis C, and HIV) from unsafe injection practices, and use of propofol vials intended for single use on multiple persons. DIPRIVAN Injectable Emulsion vials are never to be accessed more than once or used on more than one person.
(See WARNINGS and DOSAGE AND ADMINISTRATION,Handling Procedures).
Anesthesia S/I-50 Description
DIPRIVANR (Propofol) Injectable Emulsion, USP is a sterile, nonpyrogenic emulsion containing 10 mg/mL of propofol suitable for intravenous administration. Propofol is chemically described as 2,6-diisopropylphenol. The structural formula is:
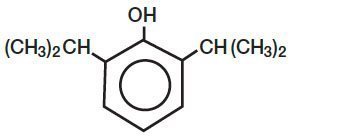
DIPRIVAN
C12H18O M.W. 178.27
Propofol is slightly soluble in water and, thus, is formulated in a white, oil-in-water emulsion. The pKa is 11. The octanol/water partition coefficient for propofol is 6761:1 at a pH of 6 to 8.5. In addition to the active component, propofol, the formulation also contains soybean oil (100 mg/mL),...



