Generic name: lidocaine hydrochloride
Dosage form: gel
Drug class:Topical anesthetics
Medically reviewed by Drugs.com. Last updated on Jan 24, 2022.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
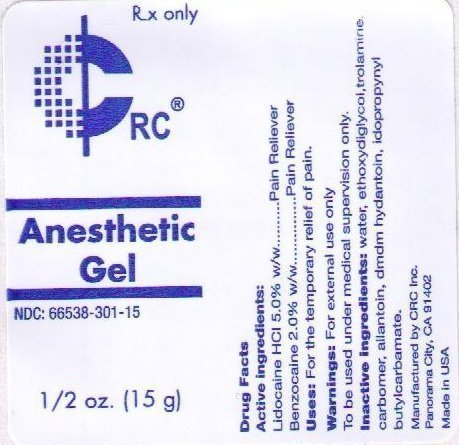
ACTIVE INGREDIENTS:
LIDOCAINE HCL 5.0% W/W
PURPOSE:
PAIN RELIEVER
On This Page
USES: FOR THE TEMPORARY RELIEF OF PAIN.
TO BE USED UNDER MEDICAL SUPERVISION ONLY.
INACTIVE INGREDIENTS: WATER, ETHOXYDIGLYCOL, TROLAMINE, CARBOMER, ALLANTOIN, DMDM HYDANTOIN, IODOPROPYNYL BUTYLCARBAMATE, ETHYL AMINOBENZOATE.
RX ONLY
CRC
Anesthetic Gel
NDC: 66538-301-15
1/2 OZ. (15 ML)
KEEP OUT OF REACH OF CHILDREN.
| ANESTHETIC lidocaine hydrochloride benzocaine gel | ||||||||||||
| ||||||||||||
| ||||||||||||



