Nordette
Generic name:levonorgestrel and ethinyl estradiol
Dosage form: tablets
Drug class:Contraceptives
Medically reviewed by Drugs.com. Last updated on Oct 22, 2021.
On This Page
Rx only
Patients should be counseled that this product does not protect against HIV infection (AIDS) and other sexually transmitted diseases.
The Nordette brand name has been discontinued in the U.S. If generic versions of this product have been approved by the FDA, there may be generic equivalents available.
Nordette Description
21 light-orange Nordette®-28 tablets, each containing 0.15 mg of levonorgestrel, (d(-)-13 beta-ethyl-17-alpha-ethinyl-17-beta-hydroxygon-4-en-3-one), a totally synthetic progestogen, and 0.03 mg of ethinyl estradiol, (19-nor-17α-pregna-1,3,5 (10)-trien-20-yne-3,17-diol), and 7 pink inert tablets. The inactive ingredients present are cellulose, D&C Red 30, FD&C Yellow 6, lactose, magnesium stearate, and polacrilin potassium.
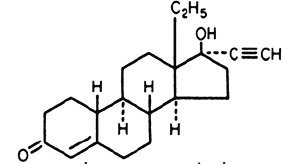
Levonorgestrel
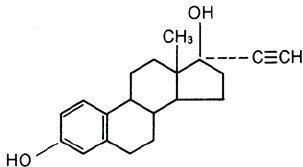
Ethinyl Estradiol
Nordette - Clinical Pharmacology
Combination oral contraceptives act by suppression of gonadotropins. Although the primary mechanism of this action is inhibition of ovulation, other alterations include changes in the cervical mucus (which increase the difficulty of sperm entry into the uterus) and the endometrium (which reduce the likelihood of implantation).
Indications and Usage for Nordette
Oral contraceptives are indicated for the prevention of pregnancy in ...



