Norlyroc
Generic name:norethindrone
Dosage form: tablet, film coated
Drug classes:Contraceptives, Progestins
Medically reviewed by Drugs.com. Last updated on Jan 24, 2022.
On This Page
Patients should be counseled that oral contraceptives do not protect against transmission of HIV (AIDS) and other sexually transmitted diseases (STDs) such as Chlamydia, genital herpes, genital warts, gonorrhea, hepatitis B, and syphilis.
Norlyroc Description
Each white Norlyroc tablet, USP provides a continuous oral contraceptive regimen of 0.35 mg norethindrone, USP daily, and the inactive ingredients include hydrogenated cottonseed oil, hydroxypropyl cellulose, hypromellose, lactose monohydrate, magnesium stearate, povidone, pregelatinized starch, talc, and titanium dioxide.
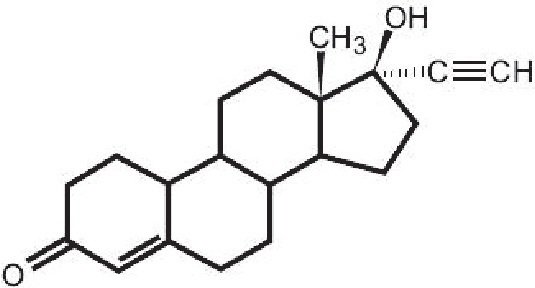
Norethindrone, USP is a white to creamy white, odorless, crystalline powder practically insoluble in water; soluble in chloroform and in dioxane; sparingly soluble in alcohol; slightly soluble in ether.
The chemical name for norethindrone is 17-Hydroxy-19-Nor-17α-pregn-4-en-20-yn-3-one. The structural formula follows:
Therapeutic class = oral contraceptive.
Norlyroc - Clinical Pharmacology
1. Mode of Action. Norlyroc progestin-only oral contraceptives prevent conception by suppressing ovulation in approximately half of users, thickening the cervical mucus to inhibit sperm penetration, lowering the mid-cycle LH and FSH peaks, slowing the movement of the ovum through the fallopian tubes, and altering the endometrium.
Absorption: Norethindrone is rapidly absorbed with maximum plasma concentrations occurring within 1 to 2 hours after norethi...