Normosol-R and Dextrose
Generic name: dextrose, sodium chloride, sodium acetate anhydrous, sodium gluconate, potassium chloride and magnesium chloride
Dosage form: injection, solution
Drug class:Intravenous nutritional products
Medically reviewed by Drugs.com. Last updated on Oct 1, 2021.
On This Page
MULTIPLE ELECTROLYTES AND 5% DEXTROSE INJECTION TYPE 1, USP
For Replacing Acute Losses of Extracellular Fluid
Flexible Plastic Container
Rx only
Normosol-R and Dextrose Description
Normosol-R and 5% Dextrose Injection is a sterile, nonpyrogenic solution of balanced electrolytes (with dextrose) in water for injection. The solution is administered by intravenous infusion for parenteral replacement of acute losses of extracellular fluid (with minimal carbohydrate calories).
Each 100 mL of Normosol-R and 5% Dextrose Injection contains dextrose 5 g; sodium chloride 526 mg; sodium acetate, anhydrous 222 mg; sodium gluconate 502 mg; potassium chloride 37 mg; magnesium chloride, hexahydrate 30 mg; pH adjusted with hydrochloric acid.
See TABLE for summary of electrolyte content, caloric value and characteristics of this solution.
The solution contains no bacteriostat, antimicrobial agent or added buffer (except for pH adjustment) and is intended only for use as a single-dose injection. When smaller doses are required the unused portion should be discarded.
Normosol-R and 5% Dextrose Injection is a parenteral fluid, electrolyte and nutrient replenisher.
Dextrose, USP is chemically designated D-glucose monohydrate (C6H12O6 • H20), a hexose sugar freely soluble in water. It has the following structural formula:
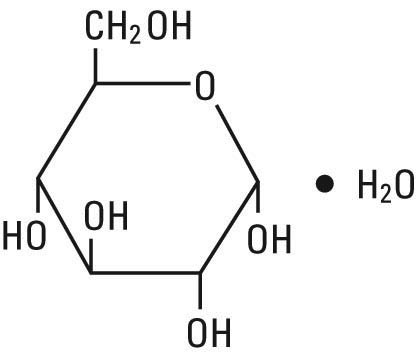
Sodium Chloride, USP is chemically designated NaCl, a white crystalline powder freely soluble in water.
Potassium Chloride, USP is chemically designated KCl, a white granular powder freely soluble in water.
Magnesium Chloride, USP is chemically designated magnesium chloride hexahydrate (MgCl2 • 6H2O...



