Normosol-R Injection
Generic name:sodium chloride, sodium acetate anhydrous, sodium gluconate, potassium chloride, and magnesium chloride
Dosage form: injection, solution
Drug class:Intravenous nutritional products
Medically reviewed by Drugs.com. Last updated on Jan 1, 2021.
On This Page
MULTIPLE ELECTROLYTES INJECTION TYPE 1, USP
For Replacing Acute Losses of Extracellular Fluid
Flexible Plastic Container
Rx only
Normosol-R Injection Description
Normosol-R is a sterile, nonpyrogenic isotonic solution of balanced electrolytes in water for injection. The solution is administered by intravenous infusion for parenteral replacement of acute losses of extracellular fluid.
Each 100 mL of Normosol-R contains sodium chloride, 526 mg; sodium acetate, 222 mg; sodium gluconate, 502 mg; potassium chloride, 37 mg; magnesium chloride hexahydrate, 30 mg. May contain HCl and/or NaOH for pH adjustment. pH 6.6 (4.0 to 8.0); 294 mOsmol/liter (calc.).
Electrolytes per 1000 mL (not including pH adjustment): Sodium 140 mEq; potassium 5 mEq; magnesium 3 mEq; chloride 98 mEq; acetate 27 mEq; gluconate 23 mEq.
The solution contains no bacteriostat, antimicrobial agent or added buffer (except for pH adjustment) and is intended only for use as a single-dose injection. When smaller doses are required the unused portion should be discarded.
Normosol-R is a parenteral fluid and electrolyte replenisher.
Sodium Chloride, USP is chemically designated NaCl, a white crystalline powder freely soluble in water.
Potassium Chloride, USP is chemically designated KCl, a white granular powder freely soluble in water.
Magnesium Chloride, USP is chemically designated magnesium chloride hexahydrate (MgCl2 • 6H2O) deliquescent crystals very soluble in water.
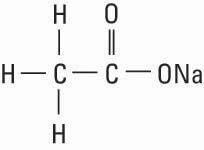
Sodium Acetate, USP, is chemically designated sodium acetate anhydrous (C2H3NaO2), a hygroscopic powder soluble in water. It has the following structural formula:
Sodium gluconate is chemically designated C6



