Obag Nu-Derm Clear
Generic name: hydroquinone
Dosage form: cream, lotion
Drug class:Topical depigmenting agents
Medically reviewed by Drugs.com. Last updated on Oct 22, 2021.
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
On This Page
Rx Only
For external use only
Obag Nu-Derm Clear Description
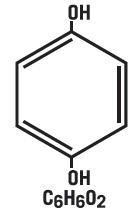
Hydroquinone is 1, 4-benzenediol. The drug is freely soluble in water and in alcohol. Chemically, hydroquinone is designed as p-dihydroxybenzene; the empirical formula is C6H6O2; molecular weight is 110.11 g/mol. The chemical structure is in the diagram below.
Each gram of Obagi Nu-Derm Clear contains:
Active ingredient: Hydroquinone USP, 4% (40 mg/g)
Inactive ingredients: water, cetyl alcohol, glycerin, sodium lauryl sulfate, stearyl alcohol, lactic acid, tocopheryl acetate, ascorbic acid, sodium metabisulfite, disodium EDTA, methylparaben, BHT, propylparaben, saponins, butylparaben
Each gram of Obagi Nu-Derm Blender contains:
Active ingredient: Hydroquinone USP, 4% (40 mg/g)
Inactive ingredients: water, glycerin, cetyl alcohol, PPG-2 myristyl ether propionate, sodium lauryl sulfate, TEA-salicylate, lactic acid, phenyl trimethicone, tocopheryl acetate, sodium metabisulfite, ascorbic acid, methylparaben, disodium EDTA, propylparaben, saponins, BHT
Each gram of Obagi Nu-Derm Sunfader contains:
Active ingredients: Hydroquinone USP, 4% (40 mg/g); Octinoxate USP, 7.5%; Oxybenzone USP, 5.5%
Inactive ingredients: water, cetyl alcohol, glycerin, sodium lauryl sulfate, stearyl alcohol, tocopheryl acetate, ascorbic acid, sodium metabisulfite, disodium EDTA, methylparaben, BHT, saponins, propylparaben, butylparaben
Obag Nu-Derm Clear - Clinical Pharmacology
Topical application of hydroquinone produces a reversible depigmentation of the skin...