OctreoScan
Generic name:indium In -111 pentetreotide
Dosage form: injection
Drug class:Diagnostic radiopharmaceuticals
Medically reviewed by Drugs.com. Last updated on Feb 1, 2022.
On This Page
OctreoScan Description
OctreoScan™ is a kit for the preparation of Indium In 111 Pentetreotide Injection, a radioactive diagnostic agent. It is a kit consisting of two components:
1) A 10-mL OctreoScan Reaction Vial which contains a lyophilized mixture of:
(i) 10 mcg pentetreotide [N-(diethylenetriamine-N,N,N′,N″-tetraacetic acid-N″-acetyl)-D-phenylalanyl-L-hemicystyl-L-phenylalanyl-D-tryptophyl-L-lysyl-L-threonyl-L-hemicystyl-L-threoninol cyclic (2→7) disulfide], (also known as octreotide DTPA),
(ii) 2 mg gentisic acid [2, 5-dihydroxybenzoic acid],
(iii) 4.9 mg trisodium citrate, anhydrous,
(iv) 0.37 mg citric acid, anhydrous, and
(v) 10 mg inositol.
Pentetreotide has the following structural formula:
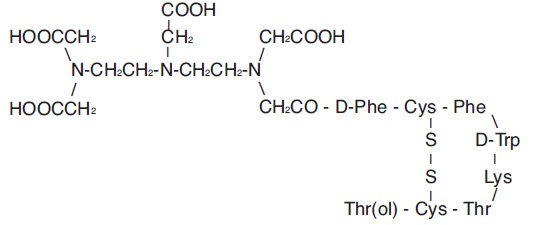
Prior to lyophilization, sodium hydroxide or hydrochloric acid may have been added for pH adjustment. The vial contents are sterile and nonpyrogenic. No bacteriostatic preservative is present.
2) A 10-mL vial of Indium In 111 Chloride Solution, which contains: 1.1 mL or 111 MBq/mL (3 mCi/mL) indium In 111 chloride in 0.02N HCl at time of calibration. The vial also contains ferric chloride at a concentration of 3.5 mcg/mL (ferric ion, 1.2 mcg/mL). The vial contents are sterile and nonpyrogenic. No bacteriostatic preservative is present.
Indium In 111 Pentetreotide Injection is prepared by combining the two kit components (seeINSTRUCTIONS FOR THE PREPARATION OF INDIUM In 111 PENTETREOTIDE INJECTION). Indium In-111 reacts with the diethylenetriaminetetraacetic acid portion of the pentetreotide molecule to form indium In 111 pentetreotide. The pH of the resultant Indium In 111 Pentetreotide Injection is between 3.8 and 4.3. No bacteriostatic preservative is present.
The Indium In 111 Pentetreotide Injection is suitable for intravenous administration as is, or it may be diluted to a maximum volume of 3 mL with 0.9% Sodium Chloride Injection, USP, immediately before intravenous administration. In either case, the radiolabeling yield of Indium In 111 Pentetreotide Injection should be determined before administration to the patient. A method r



