Penicillamine Capsules
Dosage form: capsule
Drug class:Antirheumatics
Medically reviewed by Drugs.com. Last updated on Oct 1, 2021.
On This Page
Physicians planning to use penicillamine should thoroughly familiarize themselves with its toxicity, special dosage considerations, and therapeutic benefits. Penicillamine should never be used casually. Each patient should remain constantly under the close supervision of the physician. Patients should be warned to report promptly any symptoms suggesting toxicity.
Penicillamine Capsules Description
Penicillamine, USP is a chelating agent used in the treatment of Wilson's disease. It is also used to reduce cystine excretion in cystinuria and to treat patients with severe, active rheumatoid arthritis unresponsive to conventional therapy (see INDICATIONS). It is 3-mercapto-D-valine. It is a white, or practically white, crystalline powder, freely soluble in water, slightly soluble in alcohol, and insoluble in ether, acetone, benzene, and carbon tetrachloride. Although its configuration is D, it is levorotatory as usually measured:
| [α] | 25° | = -62.5° ± 2° (c = 1, 1N NaOH), |
| D | ||
calculated on a dried basis.
The empirical formula is C5H11NO2S, giving it a molecular weight of 149.21. The structural formula is:
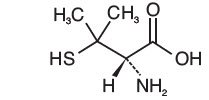
It reacts readily with formaldehyde or acetone to form a thiazolidine-carboxylic acid. Penicillamine Capsules, USP for oral administration contain 250 mg of penicillamine. Each capsule contains the following inactive ingredients: D&C Yellow No. 10, gelatin, lactose monohydrate, magnesium stearate, titanium dioxide, shellac, black iron oxide, and potassium hydroxide. "FDA approved dissolution test differs from the USP".
Penicillamine Capsules - Clinical Pharmacology
Penicillamine is a chel...



