Physostigmine Injection
Generic name: physostigmine salicylate
Dosage form: injection
Drug class:Antidotes
Medically reviewed by Drugs.com. Last updated on Nov 1, 2021.
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. Read further information about unapproved drugs.
On This Page
Physostigmine Injection Description
Physostigmine Salicylate Injection is a derivative of the Calabar bean, and its active moiety, physostigmine, is also known as eserine. Its chemical structure is:
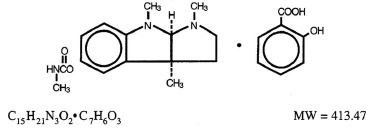
It is soluble in water and a 0.5% aqueous solution has a pH of 5.8.
Physostigmine Salicylate Injection is available in 2 mL ampules, each mL containing 1 mg of Physostigmine Salicylate in a vehicle composed of sodium metabisulfite 0.1%, benzyl alcohol 2.0% as a preservative in Water for Injection.
Physostigmine Injection - Clinical Pharmacology
Physostigmine Salicylate Injection is a reversible anticholinesterase which effectively increases the concentration of acetylcholine at the sites of cholinergic transmission. The action of acetylcholine is normally very transient because of its hydrolysis by the enzyme, acetylcholinesterase. Physostigmine Salicylate Injection inhibits the destructive action of acetylcholinesterase and thereby prolongs and exaggerates the effect of the acetylcholine.
Physostigmine Salicylate Injection contains a tertiary amine and easily penetrates the blood brain barrier, while an anticholinesterase, such as neostigmine, which has a quaternary ammonium ion is not capable of crossing the barrier. Physostigmine Salicylate Injection can reverse both central and peripheral anticholinergia. The anticholinergic syndrome has both central and peripheral signs and symptoms. Central toxic effects include anxiety, delirium, disorientation, hallucinations, hyperactivity and seizures. Severe poisoning may produce coma, medullary paralysis and death. Peripheral toxicity is characterized by tachycardia, hyperpyrexia, mydriasis, vasodilation, urinary retention, diminution of gastrointestinal motility, decrease of secretion in salivary and sweat glands, and loss of secretions in the pharynx, bronchi, and nasal passages.
Dramatic reversal of the effects of anticholinergic symptoms can be expected in minutes after the intravenous administration of Physostigm...



