Pigment Control Program - Hydroquinone
Generic name: hydroquinone
Dosage form: kit
Drug class:Topical depigmenting agents
Medically reviewed by Drugs.com. Last updated on Apr 1, 2022.
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. Read further information about unapproved drugs.
On This Page
PIGMENT CONTROL CREME
(Hydroquinone USP, 4%)
RX ONLY
FOR EXTERNAL USE ONLY:
NOT FOR OPHTHALMIC USE
Pigment Control Program - Hydroquinone Description
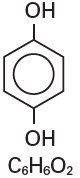
Hydroquinone is 1,4-benzendiol, with a chemical formula of C6H6O2 and a molecular weight of 110.11.
The structural formula is:
Each gram of Pigment Control Creme (Hydroquinone USP, 4%) contains Hydroquinone USP 40 mg/gm in a base of Purified Water, Ascorbic Acid, Ascorbyl Palmitate, Beta-Glucan, Caprylyl Glycol, Cetyl Alcohol, Chlorphenesin, Dioscorea Villosa (Wild Yam) Root Extract, Disodium EDTA, Glycerin, Glycolic Acid, Phenoxyethanol, Quillaja Saponaria Bark Extract, Smilax Aristolochiifolia Root Extract, Sodium Hydroxide, Sodium Lauryl Sulfate, Sodium Metabisulfite, Sodium Sulfite, Stearyl Alcohol, Tocopheryl Acetate, Yucca Schidigera Root Extract.
Pigment Control Program - Hydroquinone - Clinical Pharmacology
Topical application of hydroquinone produces a reversible depigmentation of the skin by inhibition of the enzymatic oxidation of tyrosine to 3,4-dihydroxyphenylalanine (DOPA) and suppression of other melanocyte metabolic processes. Exposure to sunlight or ultraviolet light will cause re-pigmentation of bleached areas, which may be prevented by the use of the sunscreen agents.
Indications and Usage for Pigment Control Program - Hydroquinone
Pigment Control Creme is indicated in the gradual bleaching of hyperpigmentation, skin conditions such as chloasma, melasma, freckles, senile lentigines, and other unwanted areas of melanin hyperpigmentation.
Contraindications
Prior history of sensitivity or allergic reaction to this product or any o.



