Poly-Tussin EX Syrup
Generic name: dihydrocodeine bitartrate, phenylephrine hydrochloride and guaifenesin
Dosage form: syrup
Drug class:Upper respiratory combinations
Medically reviewed by Drugs.com. Last updated on Oct 22, 2021.
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
On This Page
Poly-Tussin EX Syrup
Rx OnlyCIII
DESCRIPTION
Each 5 mL (one teaspoonful) for oral administration contains:
Dihydrocodeine Bitartrate..... 7.5 mg
(WARNING- May be habit forming)
Phenylephrine HCl..... 5 mg
Guaifenesin..... 50 mg
This product contains the following inactive ingredients:
Purified Water, Propylene Glycol, Citric Acid, Sodium Citrate, Sodium Saccharin, Glycerin, Sorbitol,
Grape Flavor, Bitter Mask.
This product contains ingredients of the following therapeutic classes: Antitussive, Decongestant and
Expectorant.
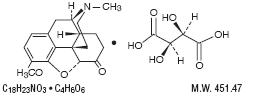
Dihydrocodeine Bitartrate is an antitussive with the chemical name Morphinan-6-ol,4,5-epoxy-3-methoxy-
17-methyl-, (5α, 6α)-2,3-dihydroxybutanedioate (1:1) (salt). It has the following structural formula:
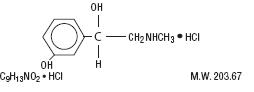
Phenylephrine hydrochloride is an orally effective nasal decongestant. Chemically it is benzenemethanol,
3-hydroxy-α-[(methylamino)methyl]-, hydrochloride (R)-. Its chemical structure is as follows:
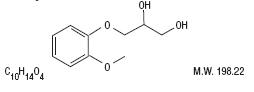
Guaifenesin is an expectorant with the chemical name 1, 2-Propanediol, 3-(2-methoxyphenoxy)-(±)-. It has
the following structural formula: