Portia
Generic name:levonorgestrel and ethinyl estradiol
Dosage form: tablets
Drug class:Contraceptives
Medically reviewed by Drugs.com. Last updated on Feb 1, 2022.
On This Page
WARNING: CIGARETTE SMOKING AND SERIOUS CARDIOVASCULAR EVENTS
Cigarette smoking increases the risk of serious cardiovascular events from combination oral contraceptive (COC) use. This risk increases with age, particularly in women over 35 years of age, and with the number of cigarettes smoked. For this reason, COCs, including Portia, are contraindicated in women who are over 35 years of age and smoke (see CONTRAINDICATIONS and WARNINGS (1)).
Portia Description
Portia® (levonorgestrel and ethinyl estradiol tablets USP) is a combination oral contraceptive (COC) consisting of 21 pink active tablets, each containing 0.15 mg of levonorgestrel, USP, a synthetic progestin and 30 mcg of ethinyl estradiol, USP, an estrogen, and 7 white inert tablets (without hormones).
The structural formulas for the active components are:
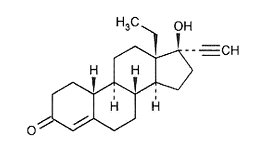
Levonorgestrel, USP
C21H28O2 M.W. 312.45
Levonorgestrel, USP is chemically 18,19-Dinorpregn-4-en-20-yn-3-one, 13-ethyl-17-hydroxy-,(17α)-(-)-.
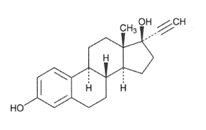
Ethinyl Estradiol, USP
C20H24O2 M.W. 296.40
Ethinyl Estradiol, USP is 19-nor-17α-pregna-1,3,5(10)-trien-20-yne-3, 17-diol.
Each pink active tablet contains the following inactive ingredients: anhydrous lactose, hypromellose, magnesium stearate, and microcrystalline cellulo...



