Positive Skin Test Control - Histamine
Generic name: histamine
Dosage form: injection
On This Page
For Percutaneous (Scratch, Prick or Puncture) Administration
Histamine Base: 6mg/mL
(Histamine Dihydrochloride: 10mg/mL)
This product is to be used by a physician or under the supervision of a physician.
Positive Skin Test Control - Histamine Description
Histamine Dihydrochloride contains histamine, a potent vasodilator having the chemical name 2-(4-Imidazolyl) ethylamine. Histamine has an empirical formula of C5H9N3, a molecular weight of 111.15, and the following chemical structure:
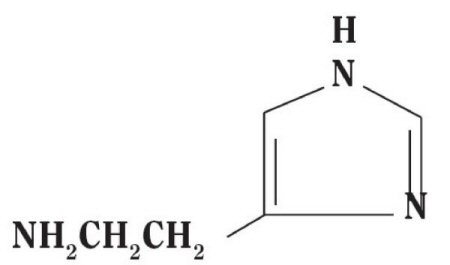
Histamine Dihydrochloride is available in the following strengths:
1. SCRATCH, PRICK or PUNCTURE TEST CONTROL:
Positive Skin Test Control - Histamine contains 6.0 mg/mL Histamine Base and is a clear, colorless, sterile solution. It consists of Histamine Dihydrochloride 10mg/mL, Sodium Chloride 0.5%, Sodium Bicarbonate 0.275%, and Glycerin 50.0% (v/v) as a preservative.
Positive Skin Test Control - Histamine - Clinical Pharmacology
Pharmacological actions of histamine include the increase of capillary and post capillary venular permeability. This vascular change leads to the wheal-flare response. Large reactions can cause an amplification of a reaction to a nearby skin test. Histamine is degraded either by oxidative deamination or by methylation and oxidative deamination so that the principal excretion products are imidazoleacetic acid-riboside and 1-methyl imidazoleacetic acid respectively.2
10 atopic subjects and 10 non-atopics were tested with Positive Skin Test Control - Histamine Solutions, and two negative control solutions. Thirteen females (35-49 years old; mean age 36.5) and 7 males (23-45 years old; mean age 36.4) were tested. This study included 19 Caucasian subjects and 1 African American subject. Different skin test devices produce different skin test responses. (



