Primacare
Generic name:prenatal/postnatal multivitamin, mineral, essential fatty acids
Dosage form: softgel
Drug classes:Iron products, Vitamin and mineral combinations
Medically reviewed by Drugs.com. Last updated on Feb 21, 2022.
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
On This Page
DESCRIPTION: Primacare™ is a prescription prenatal/postnatal multivitamin/mineral/essential fatty acid softgel. Each softgel is purple in color and imprinted with “PRIMA” on one side and blank on the other.'
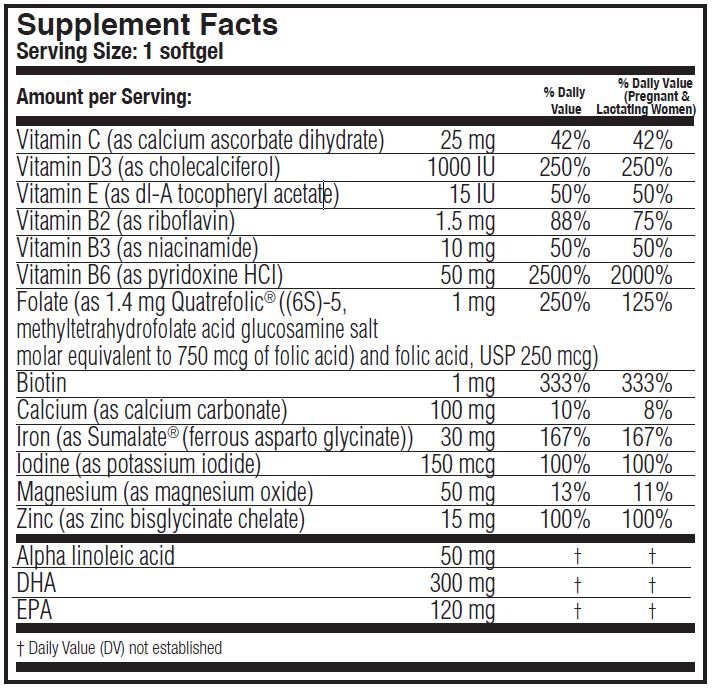
Primacare™ contains flaxseed oil, fish oil and soy.
OTHER INGREDIENTS: Gelatin capsule (bovine gelatin, sorbitol, glycerin, purified water, titanium dioxide, FD&C Red #40, caramel coloring, FD&C Blue #1), yellow beeswax and soy lecithin.
INDICATIONS: Primacare™ is a multivitamin/multimineral fatty acid dietary supplement indicated for use in improving the nutritional status of omen throughout pregnancy and in the postnatal period for both lactating and nonlactating mothers.
CONTRAINDICATIONS: Primacare™ is contraindicated in patients with a known hypersensitivity to any of the ingredients.
WARNING: Ingestion of more than 3 grams of omega-3 fatty acids (such as DHA) per day has been shown to have potential antithrombotic effects, including an increased bleeding time and International Normalized Ratio (INR). Administration of omega-3 fatty acids should be avoided n patients taking anticoagulants and in those known to have an inherited or acquired predisposition to bleeding.
PRECAUTIONS: Folic acid alone is improper therapy in
the treatment of pernicious anemia and other
megaloblastic anemias where Vitamin B12 is deficient.
Folic acid in doses above 1



