Primsol Oral Solution
Generic name:trimethoprim hydrochloride
Dosage form: oral solution
Drug class:Urinary anti-infectives
Medically reviewed by Drugs.com. Last updated on Dec 1, 2020.
Dye-free, alcohol-free, flavored solution,
50 mg trimethoprim per 5 mL
On This Page
Primsol Oral Solution Description
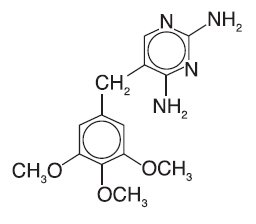
PRIMSOL (trimethoprim hydrochloride oral solution) is a solution of the synthetic antibacterial trimethoprim in water prepared with the aid of hydrochloric acid. Each 5 mL for oral administration contains trimethoprim hydrochloride equivalent to 50 mg trimethoprim and the inactive ingredients bubble gum flavor, fructose, glycerin, methylparaben, monoammonium glycyrrhizinate, povidone, propylparaben, propylene glycol, saccharin sodium, sodium benzoate, sorbitol, water and hydrochloric acid and/or sodium hydroxide to adjust pH to a range of 3.0 - 5.0. Trimethoprim is 2,4-diamino-5- (3,4,5-trimethoxybenzyl) pyrimidine. Trimethoprim is a white to cream-colored, odorless, bitter compound with a molecular formula of C 14H 18N 4O 3 and a molecular weight of 290.32 and the following structural formula:
Primsol Oral Solution - Clinical Pharmacology
Trimethoprim is rapidly absorbed following oral administration.
It exists in the blood as unbound, protein-bound and metabolized forms. Ten to twenty percent of trimethoprim is metabolized, primarily in the liver; the remainder is excreted unchanged in the urine. The principal metabolites of trimethoprim are the 1- and 3-oxides and the 3′- and 4′- hydroxy derivatives. The free form is considered to be the therapeutically active form. Approximately 44% of trimethoprim is bound to plasma proteins.
Mean peak plasma concentrations of approximately 1 mcg/mL occur 1 to 4 hours after oral administration of a single 100 mg dose. A single 200 mg dose will result in plasma concentrations approximately twice as high. The mean half-life of trimethoprim is approximately 9 hours. However, patients with severely impaired renal function exhibit an increase in the half-life of trimethoprim, which requires either dosage regimen adjustment or not using the drug in such patients (see



