Aranelle
Generic name:norethindrone and ethinyl estradiol
Dosage form: tablets
Drug classes:Contraceptives, Sex hormone combinations
Medically reviewed by Drugs.com. Last updated on Dec 1, 2021.
On This Page
Patients should be counseled that this product does not protect against HIV infection (AIDS) and other sexually transmitted diseases.
Aranelle Description
Aranelle® 28-Day Regimen (norethindrone and ethinyl estradiol tablets USP) provides a continuous oral contraceptive regimen of 7 light yellow tablets, 9 white tablets, 5 more light yellow tablets, and then 7 peach tablets. Each light yellow tablet contains norethindrone, USP 0.5 mg and ethinyl estradiol, USP 0.035 mg, each white tablet contains norethindrone, USP 1 mg and ethinyl estradiol, USP 0.035 mg, and each peach tablet contains inert ingredients.
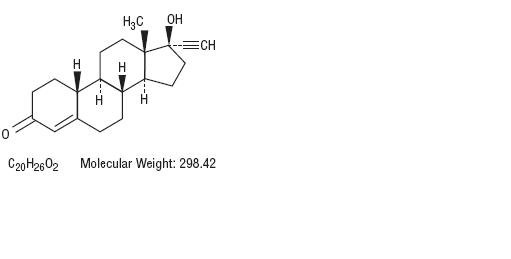
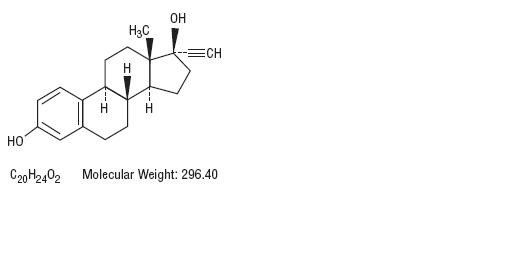
Norethindrone, USP is a potent progestational agent with the chemical name 17-Hydroxy-19-nor-17α-pregn-4-en-20-yn-3-one. Ethinyl estradiol, USP is an estrogen with the chemical name 19-Nor-17α-pregna-1,3,5(10)-trien-20-yne-3,17-diol. Their structural formulae follow.
Norethindrone, USP
Ethinyl Estradiol, USP
The light yellow tablet contains the following inactive ingredients, D&C yellow no. 10 aluminum lake, lactose monohydrate, magnesium stearate, and pregelatinized starch.
The white tablet contains the following inactive ingredients, lactose monohydrate, magnesium stearate, and pregelatinized starch.
The inactive peach tablets contain the following inactive ingredients, anhydrous lactose, FD&C yellow no. 6 aluminum lake, magnesium stearate, microcrystalline cellulose, and pregelatinized starch.
Aranelle - Clinical Pharmacology
Combination oral contrace...



