PS1 Aciurgy Pack
Generic name: mupirocin, chlorhexidine gluconate, povidone-iodine
Dosage form: kit
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. Read further information about unapproved drugs.
On This Page
For Dermatologic Use
Rx Only
-------------------- Mupirocin Ointment USP, 2% --------------------
PS1 Aciurgy Pack Description
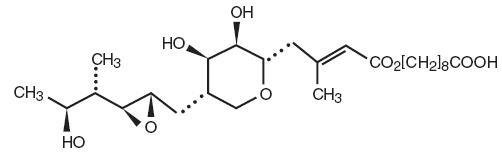
Each gram of Mupirocin Ointment USP, 2% contains 20 mg mupirocin in a bland water miscible ointment base (polyethylene glycol ointment, NF) consisting of polyethylene glycol 400 and polyethylene glycol 3350. Mupirocin is a naturally occurring antibiotic. The chemical name is ( E)-(2 S,3 R,4 R,5 S)-5-[(2 S,3 S,4 S,5 S)-2,3-Epoxy-5-hydroxy-4-methylhexyl]tetrahydro-3,4-dihydroxy-β-methyl-2 H-pyran-2-crotonic acid, ester with 9-hydroxynonanoic acid. The molecular formula of mupirocin is C 26 H 44 O 9 and the molecular weight is 500.62.
The chemical structure is:
PS1 Aciurgy Pack - Clinical Pharmacology
Application of 14C-labeled mupirocin ointment to the lower arm of normal male subjects followed by occlusion for 24 hours showed no measurable systemic absorption (<1.1 nanogram mupirocin per milliliter of whole blood). Measurable radioactivity was present in the stratum corneum of these subjects 72 hours after application.
Following intravenous or oral administration, mupirocin is rapidly metabolized. The principal metabolite, monic acid, is eliminated by renal excretion, and demonstrates no antibacterial activity. In a trial conducted in 7 healthy adult male subjects, the elimination half-life after intravenous administration of mupirocin was 20 to 40 minutes for mupiroci...



