Rectacort-HC
Generic name:hydrocortisone acetate
Dosage form: rectal suppositories
Drug class:Topical steroids
Medically reviewed by Drugs.com. Last updated on May 23, 2022.
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. Read further information about unapproved drugs.
On This Page
The Rectacort-HC brand name has been discontinued in the U.S. If generic versions of this product have been approved by the FDA, there may be generic equivalents available.
DESCRIPTION:
HYDROCORTISONE ACETATE, 25 mg
Rectal Suppositories
DESCRIPTION: Hydrocortisone Acetate is a corticosteroid designated chemically as pregn-4-ene-3, 20-dione, 21-(acetyloxy)-11, 17-dihydroxy-(11β) with the following structural formula:
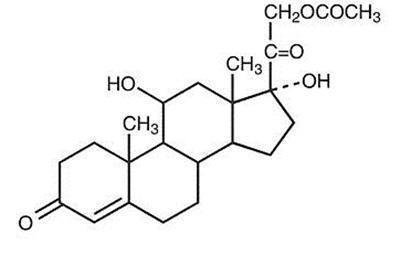
Each rectal suppository contains hydrocortisone acetate, USP 25 mg in a specially blended hydrogenated vegetable oil base.
CLINICAL PHARMACOLOGY:
In normal subjects, about 26% of hydrocortisone acetate is absorbed when the suppository is applied to the rectum. Absorption of hydrocortisone acetate may vary across abraded or inflamed surfaces. Topical steroids are primarily effective because of their anti-inflammatory, anti-pruritic and vasconstrictive action.
INDICATIONS AND USAGE:
Hydrocortisone acetate suppositories are indicated for the use in inflamed hemorrhoids, post-irradiation (factitial) proctitis; as an adjunct in the treatment of chronic ulcerative colitis; cryptitis; and other inflammatory conditions of anorectum and puritus ani.
CONTRAINDICATIONS:
Hydrocortisone acetate suppositories are indicated for use in inflamed hemorrhoids, post-irradiation (factitial) proctitis; as an adjunct in the treatment of chronic ulcerative colitis; cryptitis; and other inflammatory conditions of anorectum and pruritus ani.
PRECAUTIONS:
Do not use hydrocortisone acetate suppositories unless a adequate proctologic examination is made.
If irritation develops, the product should be discontinued and appropriate therapy instituted.
In the presen



