Sodium Iodide I 123
Dosage form: capsule, gelatin coated
Drug class:Diagnostic radiopharmaceuticals
Medically reviewed by Drugs.com. Last updated on Nov 22, 2021.
On This Page
Sodium Iodide I 123 Description
Sodium Iodide I 123 (Na123I) for diagnostic use is supplied in capsules for oral administration. The capsules are available in strengths of 3.7 and 7.4 megabecquerels (MBq) (100 and 200 μCi) I-123 at time of calibration.
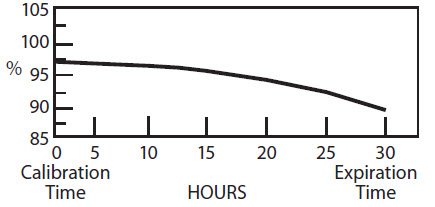
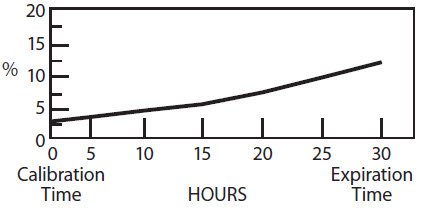
The radionuclidic composition at calibration is not less than 97.0 percent I-123, not more than 2.9 percent I-125 and not more than 0.1 percent Te-121. The radionuclidic composition at expiration time is not less than 87.2 percent I-123, not more than 12.4 percent I-125 and not more than 0.4 percent Te-121. The ratio of the concentration of I-123 and I-125 changes with time. Graph 1 shows the minimum concentration of I-123 as a function of time and Graph 2 shows the maximum concentration of I-125 as a function of time.
Graph 1. Radionuclidic Concentration of I-123
PERCENT OF TOTAL RADIOACTIVITY:
IODINE-123
Graph 2. Radionuclidic Concentration of I-125
PERCENT OF TOTAL RADIOACTIVITY:
IODINE-125
Physical Characteristics
Iodine-123 decays by electron capture with a physical half-life of 13.2 hours1. The photon that is useful for detection and imaging studies is listed in Table 1.
| Radiation | Mean % Disintegration | Energy (keV) |
| Gamma-2 |
MEDICAL DEPARTMENTS
Cardiology
Pediatrics
Diabetes Care
Pre-natal Care
Ultrasound Echocardiogram
|



