SSD Cream
Generic name:silver sulfadiazine
Dosage form: cream
Drug class:Topical antibiotics
Medically reviewed by Drugs.com. Last updated on Mar 22, 2022.
On This Page
DESCRPITION
SSD™ (1% Silver Sulfadiazine Cream) and SSD AF™ (1% Silver Sulfadiazine Cream), 1% are topical antibacterial preparations which have as their active antimicrobial ingredient silver sulfadiazine. The active moiety is contained within an opaque, white, water miscible cream base.
Each 1000 grams of SSD/SSD AF Cream contains 10 grams of silver sulfadiazine.
Inactive Ingrediants: cetyl alcohol (SSD Cream only), isopropyl myristate, polyoxyl 40 stearate, propylene glycol, purified water, stearyl alcohol, sodium hydroxide, sorbitan monooleate, white petrolatum; with 0.3% methyl paraben, as a preservative.
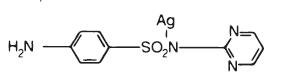
Silver sulfadiazine has an emprical formula of C10H9AgN4O2S, molecular weight of 357.14 and structural formula as shown:
SSD Cream - Clinical Pharmacology
Silver sulfadiazine has broad antimicrobial activity. It is bactericidal for many gram-negative and gram-positive bacteria as well as being effective against yeast. Results from in vitro testing are listed below. Sufficient data have been obtained to demonstrate that silver sulfadiazine will inhibit bacteria that are resistant to other antimicrobial agents and that the compound is superior to sulfadiazine. Studies utilizing radioactive micronized silver sulfadiazine, electron microscopy, and biochemical techniques have revealed that the mechanism of action of silver sulfadiazine on bacteria differs from silver nitrate and sodium sulfadiazine. Silver sulfadiazine acts only on the cell wall to produce its bactericidal effect.
Results of In Vitro Testing With Silver Sulfadiazine Cream, 1% Concentration of Silver Sulfadiazine
Number of Sensitive Strains / Total Number of Strains Tested
Genus and Species 50 micrograms/mL 100 micrograms/mL
Pseudomonas Aeruginosa 130/130 130/130
Xanthomonas (Pseudomonas)
Maltophilia 7/7 7/7
Enterobacter Species 48/50