Sting Relief
Generic name:benzocaine, alcohol
Dosage form: swab
Drug class:Topical anesthetics
Medically reviewed by Drugs.com. Last updated on Apr 21, 2022.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Active Ingredient
Medicinal Ingredient:Benzocaine6%, NON MEDICINAL: SD Alcohol 60%.Use
For the temporary relief of pain and itching associated with minor burns, scrapes and insect bitesDirections
Apply to affected area not more than 3 to 4 times daily. For adults and children 2 years of age and older. Children under 2 years: consult a physician. DO NOT USE IN EYES, broken skin, deep puncture wounds, or if unusual redness, swelling, irritation or other symptoms occur, consult a physician immediatelyCautions
FLAMMABLE.KEEP OUT OF REACH OF CHILDREN. FOR EXTERNAL USE ONLY. AVOID CONTACT WITH EYES: if this happens, rinse thoroughly with water.Inactive Ingredients
purified water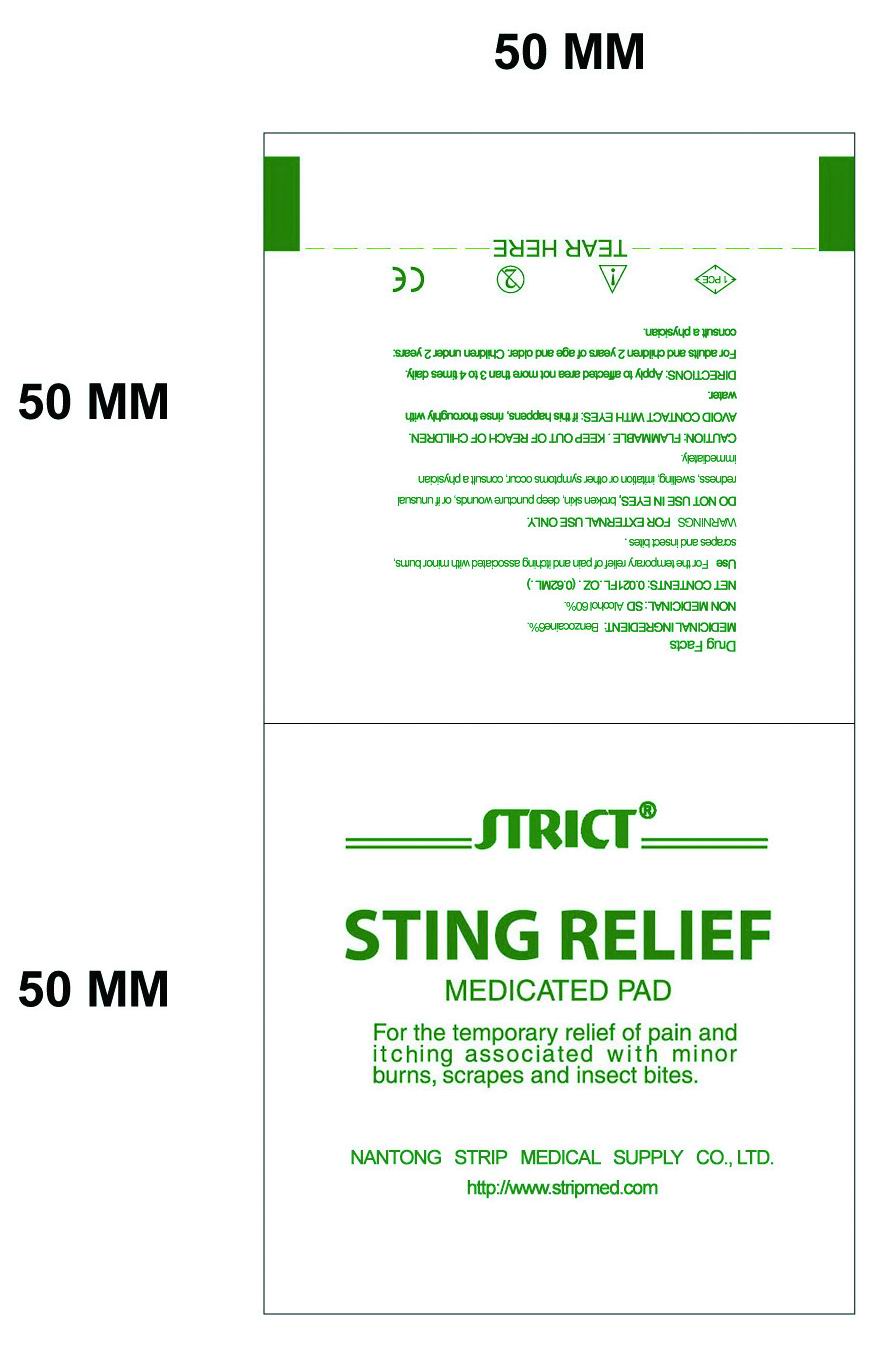
| Sting Relief benzocaine,alcohol swab | ||||||||||||
| ||||||||||||
| ||||||||||||
| ||||||||||||



