Aubra
Generic name:levonorgestrel and ethinyl estradiol
Dosage form: tablets
Drug class:Contraceptives
Medically reviewed by Drugs.com. Last updated on Jan 1, 2021.
On This Page
Aubra®
(Levonorgestrel and Ethinyl Estradiol Tablets USP, 0.1 mg/0.02 mg)
Patients should be counseled that oral contraceptives do not protect against transmission of HIV (AIDS) and other sexually transmitted diseases (STDs) such as chlamydia, genital herpes, genital warts, gonorrhea, hepatitis B, and syphilis.
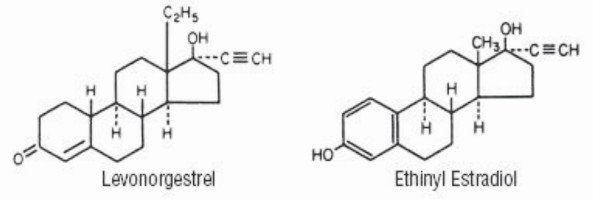
Aubra Description
Each active, light yellow tablet (21) contains 0.1 mg of levonorgestrel, d(-)-13β-ethyl-17α-ethinyl-17β-hydroxygon-4-en-3-one, a totally synthetic progestogen, and 0.02 mg of ethinyl estradiol, 17α-ethinyl-1,3,5(10)-estratriene-3,17β-diol. The inactive ingredients present are croscarmellose sodium, ferric oxide of iron (yellow), ferric oxide of iron (red), lactose monohydrate, magnesium stearate, microcrystalline cellulose, povidone K-25, and sodium lauryl sulfate.
Each inactive, brown tablet (7) contains the following inactive ingredients: croscarmellose sodium, ferric oxide of iron (brown), lactose monohydrate, magnesium stearate, microcrystalline cellulose, and povidone K-25.
Aubra - Clinical Pharmacology
Combination oral contraceptives act by suppression of gonadotropins. Although the primary mechanism of this action is inhibition of ovulation, other alterations include changes in the cervical mucus (which increase the difficulty of sperm entry into the uterus) and the endometrium (which reduce the likelihood of implantation).
Pharmacokinetics
No specific investigation of the absolute bioavailability of levonorgestrel and ethinyl estradiol tablets in humans has been conducted. However, literature indicates that levonorgestrel is rapidly and completely absorbed after oral administration (bioavailability about 100%) and is not subject to first-pass metabolism. Ethinyl estradiol is rapidly and almost completel...



