Targadox
Generic name:doxycycline hyclate
Dosage form: tablet
Medically reviewed by Drugs.com. Last updated on Jul 1, 2020.
On This Page
Rx Only
To reduce the development of drug-resistant bacteria and maintain the effectiveness of Targadox® and other antibacterial drugs, Targadox® should be used only to treat or prevent infections that are proven or strongly suspected to be caused by bacteria.
Targadox Description
Doxycycline is an antibacterial drug synthetically derived from oxytetracycline, and is available as doxycycline hyclate tablets, USP for oral administration. The chemical designation of doxycycline is 4-(Dimethylamino)-1,4,4a,5,5a,6,11,12a-octahydro-3,5,10,12,12a-pentahydroxy-6-methyl-1,11-dioxo-2-naphthacene-carboxamide monohydrochloride, compound with ethyl alcohol (2:1), monohydrate.
The structural formula of doxycycline hyclate is:
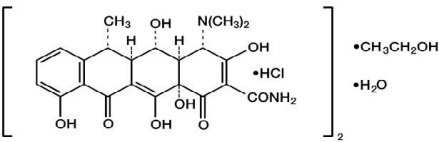
with a molecular formula of (C22H24N2O8•HCl)2•C2H6O•H2O and the molecular weight is 1025.89. Doxycycline is a light-yellow crystalline powder. Doxycycline hyclate is soluble in water.
Doxycycline has a high degree of lipoid solubility and a low affinity for calcium binding. It is highly stable in normal human serum. Doxycycline will not degrade into an epianhydro form.
Active Ingredient: Doxycycline hyclate USP equivalent to 50 mg of doxycycline.
Inactive Ingredient: Microcrystalline cellulose and magnesium stearate.
Tablet coating contains hypromellose, titanium dioxide, polyethylene glycol, FD&C yellow #6, polysorbate 80 and FD&C blue #2.
Targadox - Clinical Pharmacology
Tetracyclines are readily absorbed and are bound to plasma proteins in varying degree. They are concentrated by the liver in the bile, and excreted in the urine and feces at high concentrations and in a biologically active form. Doxycycline is virtually completely absorbed after oral administration.
Following a 200 mg dose, normal adult volunteers averaged peak serum levels of 2.6 mcg/mL...



