Generic name:norethindrone acetate and ethinyl estradiol
Dosage form: tablets
Drug class:Contraceptives
Medically reviewed by Drugs.com. Last updated on May 1, 2022.
On This Page
Aurovela 1.5/30 Description
Aurovela 1.5/30 is progestogen-estrogen combination.
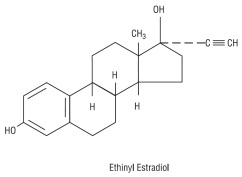
Each white to off-white tablet contains norethindrone acetate USP (17 alpha-ethinyl-19-nortestosterone acetate), 1.5 mg; ethinyl estradiol USP (17 alpha -ethinyl-1,3,5(10)-estratriene-3, 17 beta-diol), 30 mcg. Each white to off-white tablet contains the following inactive ingredients: compressible sugar, croscarmellose sodium, lactose monohydrate, magnesium stearate, microcrystalline cellulose, povidone, and vitamin E.
The structural formulas are as follows: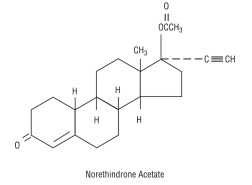
Meets USP Dissolution Test 2.
Aurovela 1.5/30 - Clinical Pharmacology
Combination oral contraceptives act by



