Generic name:norethindrone acetate and ethinyl estradiol
Dosage form: tablets
Drug class:Contraceptives
Medically reviewed by Drugs.com. Last updated on May 1, 2022.
On This Page
Patients should be counseled that this product does not protect against HIV infection (AIDS) and other sexually transmitted diseases.
Aurovela 1/20 Description
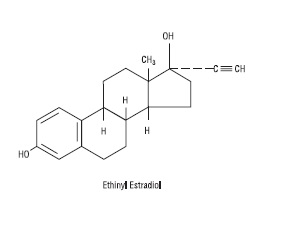
Aurovela 1/20 is progestogen-estrogen combination.
Each light yellow to yellow tablet contains norethindrone acetate USP (17 alpha-ethinyl-19-nortestosterone acetate), 1 mg; ethinyl estradiol USP (17 alpha-ethinyl-1,3,5(10)-estratriene-3, 17 beta-diol), 20 mcg. Each light yellow to yellow tablet contains the following inactive ingredients: compressible sugar, croscarmellose sodium, D&C Yellow No. 10 aluminum lake, lactose monohydrate, magnesium stearate, microcrystalline cellulose, povidone, and vitamin E.
The structural formulas are as follows: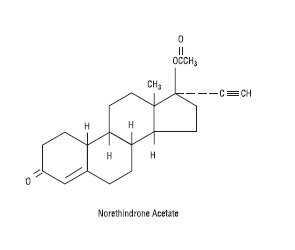
Meets USP Dissolution Test 2.



