TechneScan HDP Kit
Generic name: technetium tc 99m oxidronate
Dosage form: injection, powder, lyophilized, for solution
Drug class:Radiologic conjugating agents
Medically reviewed by Drugs.com. Last updated on Nov 22, 2021.
On This Page
TechneScan HDP Kit Description
Technescan™ HDP is supplied as a lyophilized powder, packaged under nitrogen in vials for intravenous administration after reconstitution with ADDITIVE-FREE sodium pertechnetate Tc 99m. Each vial contains 3.15 mg oxidronate sodium and 0.258 mg, minimum, stannous chloride (SnCl2•2H2O), 0.297 mg, theoretical, stannous chloride (SnCl2•2H2O) with 0.343 mg, maximum, tin chloride [stannous and stannic] dihydrate as SnCl2•2H2O as active ingredients. In addition, each vial contains 0.84 mg gentisic acid as a stabilizer and 30.0 mg sodium chloride. The pH is adjusted with hydrochloric acid and/or sodium hydroxide. The pH of the reconstituted drug is between 4.0 and 5.5. The contents of the vial are sterile and non-pyrogenic.
The chemical structure of oxidronate sodium is:
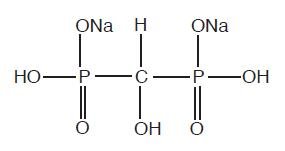
This radiopharmaceutical diagnostic agent, when reconstituted with ADDITIVE-FREE sodium pertechnetate Tc 99m forms a complex of unknown structure.
PHYSICAL CHARACTERISTICS
Technetium Tc 99m decays by isomeric transition with a physical half-life of 6.02 hours.1The principal photon that is useful for detection and imaging is listed in Table 1.
Table 1. Principal Radiation Emission Data1
Radiation | Mean Percent/ | Energy |
Gamma-2 |
MEDICAL DEPARTMENTS
Cardiology
Pediatrics
Diabetes Care
Pre-natal Care
Ultrasound Echocardiogram
|



