Technetium TC 99M Disofenin
Dosage form: injection, powder, lyophilized, for solution
Drug class:Radiologic conjugating agents
Medically reviewed by Drugs.com. Last updated on May 23, 2022.
On This Page
Technetium TC 99M Disofenin Description
Each vial contains a sterile, non-pyrogenic, lyophilized mixture of
Disofenin - 20 mg
Stannous Chloride, minimum (SnCL2•2H20) - 0.24mg
Total Tin, maximum (SnCL2•2H20) - 0.6 mg
Prior to lyophilization the pH is adjusted to between 4-5 with HCL and/or NaOH. The contents of the vial are lyophilized and stored under nitrogen.
The drug is administered by intravenous injection for diagnostic use after reconstitution with sterile, non-pyrogenic, oxidant-free Sodium Pertechnetate Tc99m Injection.
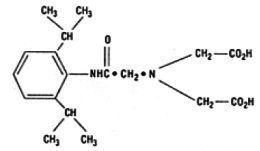
The structure of disofenin is shown below:
The precise structure of stannous technetium-disofenin complex is unknown at this time.
PHYSICAL CHARACTERISTICS
Technetium Tc99m decays by isomeric transition with a physical half-life of 6.02 hours.1 Photons that are useful for detection and imaging studies are listed in Table 1.
Table 1. Principal Radiation Emission Data | ||
Radiation | Mean % per Disintegration | Mean Energy (keV) |
Gamma-2 | 89.07 | 140.5 |
1Kocher, David C., Radioactive Decay Data Tables, DOE/TIC-11026, 108 (1981).
External Radiation
The specific gamma...