Technetium TC 99M Medronate
Dosage form: injection, powder, lyophilized, for solution
On This Page
DESCRIPTION
Each reaction vial contains a sterile, nonpyrogenic, nonradioactive lyophilized mixture of 20 mg medronic acid, 1 mg ascorbic acid, 0.13 mg (minimum) stannous fluoride, SnF2 and 0.38 mg total tin, maximum (as stannous fluoride, SnF2).
The pH is adjusted with sodium hydroxide or hydrochloric acid to 6.5 (6.3 to 6.7) prior to lyophilization. The vial does not contain a preservative. The contents of the vial are lyophilized and sealed under nitrogen at the time of manufacture.
The pH of the reconstituted product is 5.4 to 6.8.
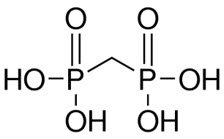
The structure of medronic acid is given below:
The precise structure of Technetium TC 99M Medronate is unknown at this time.
When sterile, pyrogen-free sodium pertechnetate Tc 99m injection is added to the vial, the diagnostic agent Technetium TC 99M Medronate is formed for administration by intravenous injection.
PHYSICAL CHARACTERISTICS
Technetium Tc 99m decays by isomeric transition with a physical half-life of 6.02 hours.1 The principal photon that is useful for detection and imaging studies is shown in Table 1.
| 1 Kocher, David C: Radioactive Decay Data Tables, DOE/TlC-11026, 108, 1981 | ||
Principal Radiation Emission Data | ||
Radiation | Mean % per | Mean Energy |
Gamma-2 | 89.07 | 140.5 |