Technetium TC 99M Mertiatide
Generic name: betiatide
Dosage form: injection, powder, lyophilized, for solution
On This Page
Rx Only.
Diagnostic-For Intravenous Use
Technetium TC 99M Mertiatide Description
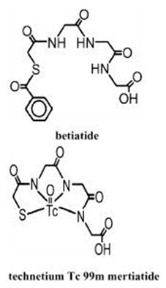
Kit for the Preparation of Technetium Tc99m Mertiatide is used for the preparation of Technetium TC 99M Mertiatide, a diagnostic radiopharmaceutical. It is supplied as a sterile, nonpyrogenic, lyophilized powder. Each vial contains betiatide (N-[N-[N-[(benzoylthio) acetyl]glycyl]glycyl]-glycine). After reconstitution with sterile sodium pertechnetate Tc 99m injection, the Technetium TC 99M Mertiatide (disodium[N-[N-[N-(mercaptoacetyl) glycyl]glycyl] glycinato (2-) - N,N′,N″,S′]oxotechnetate (2-)) which is formed is suitable for intravenous administration.
Each 10 milliliter vial contains 1 milligram betiatide, 0.05 milligram (minimum) stannous chloride dihydrate (SnCl2∙2H2O) and 0.2 milligram (maximum) total tin expressed as stannous chloride dihydrate (SnCl2∙2H2O), 40 milligrams sodium tartrate dihydrate (Na2C4H2O6∙2H2O), and 20 milligrams lactose monohydrate. Prior to lyophilization, sodium hydroxide or hydrochloric acid may be added for pH adjustment. The pH of the reconstituted drug is between 5.0 and 6.0. No bacteriostatic preservative is present. The contents are sealed under argon. Betiatide is light sensitive and must be protected from light. Betiatide and Technetium TC 99M Mertiatide have the following structural formulas:
PHYSICAL CHARACTERISTICS
Technetium Tc 99m decays by isomeric transition with a physical half-life of 6.02 hours. The principal photon that is useful for detection and imaging is listed in Table 1.
| Radiation M | Mean % per Disintegrat... |
|---|