Generic name: clobetasol propionate
Dosage form: scalp application
Drug class:Topical steroids
Medically reviewed by Drugs.com. Last updated on Jun 21, 2021.
On This Page
Rx only
FOR TOPICAL DERMATOLOGIC USE ONLY—NOT FOR OPHTHALMIC, ORAL, OR INTRAVAGINAL USE
DESCRIPTION
TEMOVATE® (clobetasol propionate scalp application) Scalp Application, 0.05% contains the active compound clobetasol propionate, a synthetic corticosteroid, for topical dermatologic use. Clobetasol, an analog of prednisolone, has a high degree of glucocorticoid activity and a slight degree of mineralocorticoid activity.
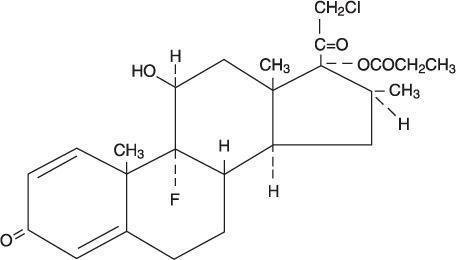
Chemically, clobetasol propionate is (11β,16β)-21-chloro-9-fluoro-11-hydroxy-16-methyl-17-(1-oxopropoxy)pregna-1, 4-diene-3,20-dione, and it has the following structural formula:
Clobetasol propionate has the molecular formula C25H32CIFO5 and a molecular weight of 467. It is a white to cream-colored crystalline powder insoluble in water.
TEMOVATE® Scalp Application contains clobetasol propionate 0.5 mg/g in a base of purified water, isopropyl alcohol (39.3%), carbomer 934R and sodium hydroxide.