Trianex Ointment
Generic name:triamcinolone acetonide
Dosage form: ointment
Drug class:Topical steroids
Medically reviewed by Drugs.com. Last updated on May 1, 2021.
On This Page
Trianex ® 0.05%
(Triamcinolone Acetonide Ointment, USP)
Proprietary Hydrous Emulsified Base
Rx Only
Trianex Ointment Description
Topical corticosteroids, such as Trianex ® 0.05% (Triamcinolone Acetonide Ointment, USP), constitute a class of primarily synthetic steroids used as anti-inflammatory and antipruritic agents.
Each gram of Trianex ® 0.05% (Triamcinolone Acetonide Ointment, USP) contains 0.5 mg of Triamcinolone Acetonide USP in a water-in-oil emulsion composed of Light Mineral Oil NF, Purified Water USP, White Petrolatum USP, Heavy Mineral Oil USP, Mineral Wax, and Lanolin Alcohols NF. The white ointment is for topical use only.
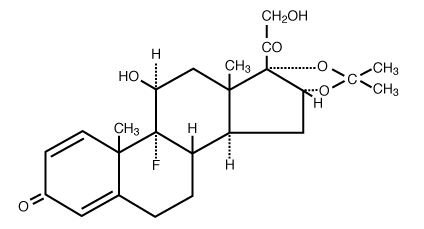
Triamcinolone Acetonide has the molecular formula of C 24H 31FO 6 and is designated chemically as Pregna-1,4-diene-3, 20-dione, 9-fluoro-11, 21-dihydroxy-16, 17-[(1-methylethylidene)bis(oxy)]-, (11β, 16α)-. It has a molecular weight of 434.50 and the following structural formula:
Trianex Ointment - Clinical Pharmacology
Topical corticosteroids share anti-inflammatory, antipruritic and vasoconstrictive actions.
The mechanism of anti-inflammatory activity of the topical corticosteroids is unclear. Various laboratory methods, including vasoconstrictor assays, are used to compare and predict potencies and/or clinical efficacies of the topical corticosteroids. There is some evidence to suggest that a recognizable correlation exists between vasoconstrictor potency and therapeutic efficacy in man.
Pharmacokinetics
The extent of percutaneous absorption of topical corticosteroids is determined by many factors including the vehicle, the integrity of the epidermal barrier, and the use of occlusive dressings.
Topical corticosteroids can be absorbed from normal intact skin. Inflammation and/or other disease processes i...



