Triaz Foaming Cloth
Generic name:benzoyl peroxide foaming cloth
Dosage form: cloth
Drug class:Topical acne agents
Medically reviewed by Drugs.com. Last updated on Feb 21, 2022.
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
On This Page
Rx Only
The Triaz brand name has been discontinued in the U.S. If generic versions of this product have been approved by the FDA, there may be generic equivalents available.
Triaz Foaming Cloth Description
TRIAZ (benzoyl peroxide) 3%, 6%, and 9% Foaming Cloths are topical preparations containing benzoyl peroxide for use in the treatment of acne vulgaris. Benzoyl peroxide is an oxidizing agent that possesses antibacterial properties and is classified as a keratolytic. Benzoyl peroxide (C14H10O4) is represented by the following chemical structure:
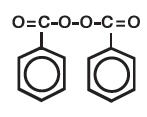
TRIAZ 3% and 6% Foaming Cloths contain, respectively, benzoyl peroxide 3% and 6% as the active ingredient in a cleanser-based formulation consisting of: purified water USP, sodium cocoyl isethionate, sodium methyl cocoyl taurate, cetyl alcohol NF, sodium lauryl sulfoacetate and disodium laureth sulfosuccinate, carbomer 1342 NF, sodium hydroxide NF, glycolic acid, hydroxypropyl methylcellulose, zinc lactate, sodium PCA, glycerin USP, docusate sodium USP, sodium hyaluronate, and simethicone USP.
TRIAZ 9% Foaming Cloths contains benzoyl peroxide 9% as the active ingredient in a cleanser-based formulation consisting of: puried water USP, sodium cocoyl isethionate, sodium methyl cocoyl taurate, cetyl alcohol NF, sodium lauryl sulfoacetate and disodium laureth sulfosuccinate, carbomer copolymer type B NF, glycolic acid, sodium hydroxide NF, hydroxypropyl methylcellulose, zinc lactate, sodium PCA, glycerin USP, docusate sodium USP, sodium hyaluronate, and simethicone USP.
Triaz Foaming Cloth - Clinical Pharmacology
The mechanism...



