Generic name: triamcinolone acetonide
Dosage form: injection, suspension
Drug class:Glucocorticoids
Medically reviewed by Drugs.com. Last updated on May 1, 2022.
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. Read further information about unapproved drugs.
On This Page
Triloan II SUIK Description
Triamcinolone acetonide injectable suspension, USP is a synthetic glucocorticoid corticosteroid with anti-inflammatory action. THIS FORMULATION IS SUITABLE FOR INTRAMUSCULAR AND INTRA-ARTICULAR USE ONLY. THIS FORMULATION IS NOT FOR INTRADERMAL INJECTION.
Each mL of the sterile aqueous suspension provides 40 mg triamcinolone acetonide, USP, with 0.65% sodium chloride for isotonicity, 0.99% (w/v) benzyl alcohol as a preservative, 0.75% carboxymethylcellulose sodium, and 0.04% polysorbate 80 in an aqueous suspension. Sodium hydroxide or hydrochloric acid may be present to adjust pH to 5.0 to 7.5. At the time of manufacture, the air in the container is replaced by nitrogen.
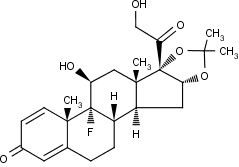
The chemical name for triamcinolone acetonide is 9-Fluoro-11β,16α,17,21-tetrahydroxypregna-1,4-diene-3,20-dione cyclic 16,17-acetal with acetone. Its structural formula is:
M...



