Ultram ODT
Generic name:tramadol hydrochloride
Dosage form: Orally Disintegrating Tablets
Drug class:Narcotic analgesics
Medically reviewed by Drugs.com. Last updated on Jul 22, 2021.
Rx Only
On This Page
The Ultram ODT brand name has been discontinued in the U.S. If generic versions of this product have been approved by the FDA, there may be generic equivalents available.
Ultram ODT Description
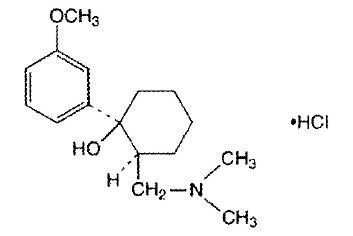
ULTRAM® ODT (tramadol hydrochloride) Orally Disintegrating Tablets is a centrally acting analgesic in an orally disintegrating formulation using a tablet formulation base. The chemical name for tramadol hydrochloride is (±) cis -2-[(dimethylamino)methyl]-1-(3-methoxyphenyl) cyclohexanol hydrochloride. Its structural formula is:
Ultram ODT is supplied as orally disintegrating tablets containing 50 mg of tramadol hydrochloride for oral administration.
Tramadol hydrochloride is a white, bitter, crystalline and odorless powder. It is readily soluble in water and ethanol and has a pKa of 9.41. The n-octanol/water log partition coefficient (logP) is 1.35 at pH 7.
The tablets are white in color and contain the inactive ingredients aspartame, copovidone, crospovidone, ethylcellulose, magnesium stearate, mannitol, mint flavor, and silicon dioxide.
Ultram ODT - Clinical Pharmacology
Pharmacodynamics
Ultram ODT is a centrally acting synthetic opioid analgesic in an orally disintegrating tablet form. Although its mode of action is not completely understood, from animal tests, at least two complementary mechanisms appear applicable: binding of parent and M1 metabolite to µ-opioid receptors and weak inhibition of reuptake of norepinephrine and serotonin.
Opioid activity is due to both low affinity binding of the parent compound and higher affinity binding of the O-demethylated metabolite M1 to µ-opioid receptors. In animal models, M1 is up to 6 times more potent than tramadol in...



