Ultrasal-ER
Generic name:salicylic acid
Dosage form: topical solution
Medically reviewed by Drugs.com. Last updated on Dec 1, 2021.
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. Read further information about unapproved drugs.
Ultrasal-ER (28.5% Salicylic Acid Extended Release) Antiviral Film-Forming Solution: Package Insert
On This Page
Rx only
FOR TOPICAL USE ONLY.
NOT FOR OPHTHALMIC, ORAL OR INTRAVAGINAL USE.
DESCRIPTION
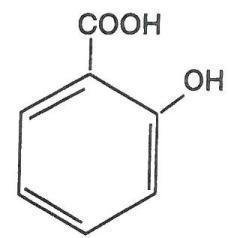
Ultrasal-ER is a topical preparation containing 28.5% salicylic acid extended release in a proprietary film-forming virucidal solution composed of acrylates copolymer, butyl acetate, carthamus tinctorius seed oil, cocamidopropyl dimethylamine, ethylhexylglycerin, isopropyl alcohol, isopropyl-metacresol, olea europaea fruit oil, phenic acid, phenoxyethanol, polysorbate 80, polyvinyl butyral, trimethyl pentanyl diisobutyrate, and water. The pharmacologic activity of Ultrasal-ER is generally attributed to the keratolytic activity of salicylic acid, which is incorporated into a polyacrylic, film-forming virucidal solution designed to cover the wart without the need for a bandage. The structural formula of salicylic acid is:
CLINICAL PHARMACOLOGY
Although the exact mode of action for salicylic acid in the treatment of warts is unknown, its activity appears to be associated with its keratolytic action, which results in mechanical removal of epidermal cells infected with wart viruses. Ultrasal-ER incorporates a unique patented extended release form of salicylic acid that provides for enhanced release of salicylic acid for over 24 hours.
The virucidal complex incorporated into Ultrasal-ER’s proprietary solution is designed to help reduce risk of reinfection at the wart site, as well as prevent viral contamination of the product under normal usage.
INDICATIONS AND USAGE
Ultrasal-ER is indicated for the topical treatment and removal of common warts and plantar warts.
CONTRAINDICATIONS
Patients with diabetes or impaired blood circulation should not use Ultrasal-ER. Ultrasal-ER also should not be used on moles, birthmarks, and unusual warts with hair growing from them, or warts on the face.
PRECAUTIONS
Ultrasal-ER is f...



