Westcort
Generic name:hydrocortisone valerate
Dosage form: cream
Drug class:Topical steroids
Medically reviewed by Drugs.com. Last updated on Aug 23, 2021.
On This Page
The Westcort brand name has been discontinued in the U.S. If generic versions of this product have been approved by the FDA, there may be generic equivalents available.
Westcort Description
Westcort® (hydrocortisone valerate ointment) Ointment, 0.2% contains hydrocortisone valerate, 11,21-dihydroxy-17-[(1-oxopentyl)oxy]-(11β)-pregn-4-ene-3,20-dione, a synthetic corticosteroid for topical dermatologic use. The corticosteroids constitute a class of primarily synthetic steroids used topically as anti-inflammatory and antipruritic agents.
Chemically, hydrocortisone valerate is C26H38O6. It has the following structural formula: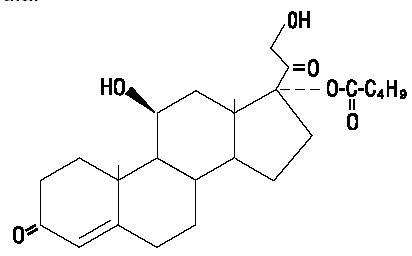
Hydrocortisone valerate has a molecular weight of 446.58. It is a white, crystalline solid, soluble in ethanol and methanol, sparingly soluble in propylene glycol and insoluble in water.
Each gram of Westcort Ointment contains 2 mg hydrocortisone valerate in a hydrophilic base composed of carbomer 934, dried sodium phosphate, mineral oil, propylene glycol, sodium lauryl sulfate, sorbic acid, steareth-2, steareth-100, stearyl alcohol, water, and white petrolatum.
Westcort - Clinical Pharmacology
Like other topical corticosteroids, hydrocortisone valerate has anti-inflammatory, antipruritic and vasoconstrictive properties. The mechanism of the anti-inflammatory activity of the topical steroids, in general, is unclear. However, corticosteroids are thought to act by the induction of phospholipase A2 inhibitory proteins, collectively called lipocortins. It is postulated that these proteins control the biosynthesis of potent mediators of inflammation such as prostaglandins and leukotrienes by inhibiting the release of their common pre...



