Zeosa
Generic name:norethindrone and ethinyl estradiol, and ferrous fumarate
Dosage form: tablets
Drug classes:Contraceptives, Sex hormone combinations
Medically reviewed by Drugs.com. Last updated on Apr 21, 2022.
On This Page
Rev. B 1/2011
Rx only
2090
Ferrous fumarate tablets are not USP for dissolution and assay.
Patients should be counseled that this product does not protect against HIV infection (AIDS) and other sexually transmitted diseases.
The Zeosa brand name has been discontinued in the U.S. If generic versions of this product have been approved by the FDA, there may be generic equivalents available.
Zeosa Description
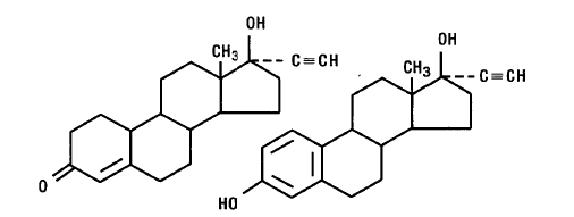
Zeosa™ norethindrone and ethinyl estradiol tablets USP (chewable) and ferrous fumarate tablets USP (chewable) provides a regimen for oral contraception derived from 21 light yellow tablets composed of norethindrone and ethinyl estradiol followed by 7 brown ferrous fumarate (placebo) tablets. The chemical name for norethindrone is 17-hydroxy-19-nor-17α-pregn-4-en-20-yn-3-one and for ethinyl estradiol the chemical name is 19-nor-17α-pregna-1,3,5(10)-trien-20-yne-3,17-diol. The structural formulas are:
NORETHINDRONEETHINYL ESTRADIOL
C20H26O2 C20H24O2
Molecular Weight: 298.42 Molecular Weight: 296.40
The active light yellow norethindrone and ethinyl estradiol tablets contain 0.4 mg norethindrone USP and 0.035 mg ethinyl estradiol USP and the following inactive ingredients: anhydrous lactose, ferric oxide, magnesium stearate, natural spearmint flavor (gum acacia and maltodextrin), pregelatinized starch and sucralose powder.
The inert brown ferrous fumarate tablets contain confectioner’s sugar, ferrous fumarate USP, magnesium stearate, microcrystalline cellulose, sodium starch glycolate, sucralose powd.