Zeyocaine
Generic name: lidocaine, isopropyl alcohol
Dosage form: kit
On This Page
FOR TOPICAL USE ONLY
DO NOT USE IN THE EYES
Rx Only
Zeyocaine Description
Lidocaine Ointment USP, 5% contains a local anesthetic agent and is administered topically. See INDICATIONS AND USAGE for specific uses.
Lidocaine Ointment USP, 5% contains lidocaine, which is chemically designated as acetamide, 2-(diethylamino)- N-(2,6-dimethylphenyl)-, and has the following structural formula:
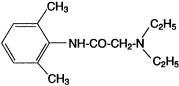
Composition of Lidocaine Ointment USP, 5%: acetamide, 2-(diethylamino)- N-(2,6-dimethylphenyl)-, (lidocaine) 5% in a water miscible ointment vehicle containing polyethylene glycol 300 and polyethylene glycol 1450.
Zeyocaine - Clinical Pharmacology
Mechanism of action
Lidocaine stabilizes the neuronal membrane by inhibiting the ionic fluxes required for the initiation and conduction of impulses, thereby effecting local anesthetic action.
Onset of anesthesia
Lidocaine Ointment USP, 5% effects local, topical anesthesia. The onset of action is 3-5 minutes. It is ineffective when applied to intact skin.
Hemodynamics
Excessive blood levels may cause changes in cardiac output, total peripheral resistance, and mean arterial pressure. These changes may be attributable to a direct depressant effect of the local anesthetic agent on various components of the cardiovascular system.
Pharmacokinetics and metabolism
Lidocaine may be absorbed following topical administration to mucous membranes, its rate and extent of absorption depending upon the specific site of application, duration of exposure, concentration, and total dosage. In general, the rate of absorption of local anesthetic agents following topical application occurs most rapidly after intratracheal administration. Lidocaine is also well-absorbed from the gastrointestinal tract, but little intact drug appears in the circulation because of biotransformation in the liver.
Lidocaine is metabolized rapidly by the liver, and metabolites and unchanged drug are excreted by the kidneys. Biotransformation includes oxidative N-dealkylation, ring hydroxylation, cleavage of the amide linkage, and conjugation. N-dealkylation, a major pathway of biotransformation, yields the metabol..



