Baycadron
Generic name:dexamethasone
Dosage form: elixir
Drug class:Glucocorticoids
Medically reviewed by Drugs.com. Last updated on Jun 21, 2021.
On This Page
The Baycadron brand name has been discontinued in the U.S. If generic versions of this product have been approved by the FDA, there may be generic equivalents available.
Baycadron Description
Each 5 mL (teaspoonful) contains:
Dexamethasone, USP……….……….…………………………. 0.5 mg
Also contains:
Benzoic Acid, USP……………………………………………….. 0.1%
(as preservative)
Alcohol……………………………………………………………. 5.1%
Inactive Ingredients: Artificial Raspberry Flavor; Citric Acid, USP; FD&C Red No. 40; Liquid Sugar; Propylene Glycol, USP and Purified Water, USP. It may also contain Sodium Citrate, USP.
Glucocorticoids are adrenocortical steroids, both naturally occurring and synthetic, which are readily absorbed from the gastrointestinal tract.
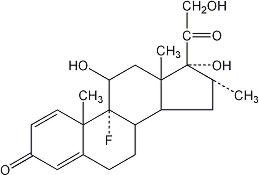
Dexamethasone, a synthetic adrenocortical steroid, is a white to practically white, odorless, crystalline powder. It is stable in air. It is practically insoluble in water. The molecular weight is 392.47. It is designated chemically as 9-fluoro-11β,17,21-trihydroxy-16α-methylpregna-1,4-diene-3,20-dione. The molecular formula is C22H29FO5 and the structural formula is:
Baycadron - Clinical Pharmacology
Naturally occurring glucocorticoids, (hydrocortisone and cortisone), which also have salt-retaining properties, are used as replacement therapy in adrenocortical deficiency states. Their synthetic analogs, including dexamethasone, are primarily used for their potent anti-inflammatory effects in disorders of many organ systems.
Glucocorticoids cause profound and varied metabolic effects. In addition, they modify the body's immune responses to diverse stimuli.
At equipotent anti-inflammatory doses, dexamethasone almost completely lacks the sodium-retaining property of hydrocortisone and closely related derivatives of hydrocortisone.
Indications and Usage for Baycadron
1. Endocrine Disorders



