Cafergot Tablets
Generic name:ergotamine tartrate and caffeine
Dosage form: tablet, film coated
Drug class:Antimigraine agents
Medically reviewed by Drugs.com. Last updated on Nov 22, 2021.
On This Page
Rx Only
Serious and/or life-threatening peripheral ischemia has been associated with the coadministration of Cafergot (ergotamine tartrate and caffeine tablets, USP) with potent CYP 3A4 inhibitors including protease inhibitors and macrolide antibiotics. Because CYP 3A4 inhibition elevates the serum levels of Cafergot (ergotamine tartrate and caffeine tablets, USP), the risk for vasospasm leading to cerebral ischemia and/or ischemia of the extremities is increased. Hence, concomitant use of these medications is contraindicated (see alsoContraindicationsandWarningssection).
Cafergot Tablets Description
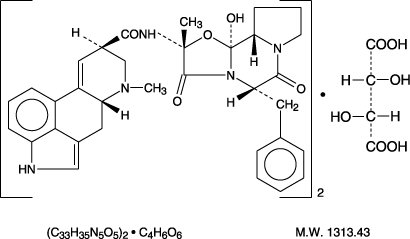
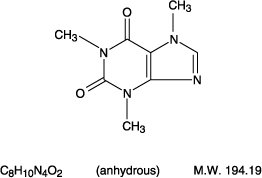
Each tablet for oral administration contains 1 mg ergotamine tartrate, USP, and 100 mg caffeine, USP.
ERGOTAMINE TARTRATE:
Ergotaman-3',6',18-trione, 12'-hydroxy-2'-methyl- 5'-(phenyl-methyl) -,(5' α) -, [ R-(R*,R*)] -2,3-dihydroxy-butanedioate (2:1) (salt).
CAFFEINE:
1H-Purine-2,6-dione, 3,7-dihydro-1,3,7- trimethyl-.
Inactive ingredients include black iron oxide, compressible sugar, iron oxide red, magnesium stearate, microcrystalline cellulose, polyethylene glycol, polyvinyl alcohol, sodium starch glycolate, talc, titanium dioxide and yellow iro...