Candesartan and Hydrochlorothiazide Tablets
Generic name: candesartan cilexetil and hydrochlorothiazide
Dosage form: tablet
Drug class:Angiotensin II inhibitors with thiazides
Medically reviewed by Drugs.com. Last updated on Apr 21, 2022.
On This Page
- •
- When pregnancy is detected, discontinue candesartan cilexetil and hydrochlorothiazide tablets as soon as possible.
- •
- Drugs that act directly on the renin-angiotensin system can cause injury and death to the developing fetus. See Warnings: Fetal Toxicity
Candesartan and Hydrochlorothiazide Tablets Description
Candesartan Cilexetil and Hydrochlorothiazide Tablets USP combine an angiotensin II receptor (type AT1) antagonist and a diuretic, hydrochlorothiazide.
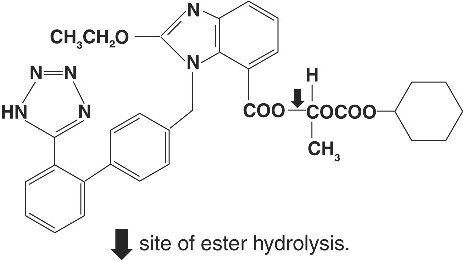
Candesartan cilexetil, a nonpeptide, is chemically described as (±)-1-Hydroxyethyl 2-ethoxy-1-[p-(o-1H-tetrazol-5-ylphenyl)benzyl]-7-benzimidazolecarboxylate, cyclohexyl carbonate (ester).
Its empirical formula is C33H34N6O6 and its structural formula is:
Candesartan cilexetil is a white to off-white powder with a molecular weight of 610.67. It is practically insoluble in water and sparingly soluble in methanol. Candesartan cilexetil is a racemic mixture containing one chiral center at the cyclohexyloxycarbonyloxy ethyl ester group. Following oral administration, candesartan cilexetil undergoes hydrolysis at the ester link to form the active drug, candesartan, which is achiral.
Hydrochlorothiazide is 6-chloro-3,4-dihydro-2H-1,2,4-benzothiadiazine-7-sulfonamide 1,1-dioxide. Its empirical formula is C7H8ClN3O4S2 and its structural formula i...