Adakveo Injection
Generic name:crizanlizumab
Dosage form: injection
Drug class:Miscellaneous uncategorized agents
Medically reviewed by Drugs.com. Last updated on Jul 1, 2021.
On This Page
- Indications and Usage
- Dosage and Administration
- Dosage Forms and Strengths
- Contraindications
- Warnings and Precautions
- Adverse Reactions/Side Effects
- Drug Interactions
- Use In Specific Populations
- Description
- Clinical Pharmacology
- Nonclinical Toxicology
- Clinical Studies
- How Supplied/Storage and Handling
- Patient Counseling Information
INDICATIONS AND USAGE
ADAKVEO® is indicated to reduce the frequency of vasoocclusive crises (VOCs) in adults and pediatric patients aged 16 years and older with sickle cell disease.
DOSAGE AND ADMINISTRATION
Recommended Dosage
Administer ADAKVEO 5 mg/kg by intravenous infusion over a period of 30 minutes at Week 0, Week 2, and every 4 weeks thereafter.
If a dose is missed, administer ADAKVEO as soon as possible.
If ADAKVEO is administered within 2 weeks after the missed dose, continue dosing according to the patient's original schedule.
If ADAKVEO is administered more than 2 weeks after the missed dose, continue dosing every 4 weeks thereafter.
ADAKVEO may be given with or without hydroxyurea.
Preparation and Administration
ADAKVEO should be prepared and administered by a healthcare professional.
Preparation
- Use aseptic technique to prepare the solution for infusion.
- Calculate the dose (mg) and the total volume (mL) of ADAKVEO solution required, and the number of ADAKVEO vials needed based on the patient’s actual body weight.
- Prepare 5 mg of ADAKVEO per kg of actual body weight.
- Calculate the volume of ADAKVEO to be used according to the following equation:
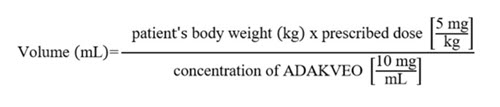
Dilution
Dilute ADAKVEO in 0.9% Sodium Chloride Injection, USP or 5% Dextrose Injection, USP to a total volume of 100 mL for intravenous infusion as follows:
- Obtain the number of vials required. One vial is needed for every 10 mL of ADAKVEO.
- Bring vials to room temperature for a maximum of 4 hours prior to the start of preparation (piercing the first vial).
- Visually inspect the vials.
- Parenteral drug products should be inspected visually f.



