Carmol HC
Generic name:hydrocortisone acetate
Dosage form: cream
Drug class:Topical steroids
Medically reviewed by Drugs.com. Last updated on Jun 21, 2021.
On This Page
RxOnly
The Carmol HC brand name has been discontinued in the U.S. If generic versions of this product have been approved by the FDA, there may be generic equivalents available.
DESCRIPTION:
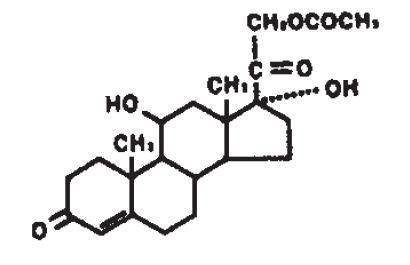
CARMOL®HC is intended for topical administration. The active component is the corticosteroid hydrocortisone acetate, which has the chemical name pregn-4-ene-3, 20-dione, 21-(acetyloxy)-11, 17-dihydroxy-, (11β)-. It has the following chemical structure.
Each gram of the cream contains 10 mg Hydrocortisone Acetate USP, in a water-washable vanishing cream base containing urea (10%), purified water, stearic acid, propylene glycol, isopropyl myristate, isopropyl palmitate, PPG-26 oleate, sodium laureth sulfate, triethanolamine, xanthan gum, sodium metabisulfite, cetyl alcohol, edentate disodium, carbomer with hypoallergenic perfume. It is nonocclusive, and contains no mineral oil, petrolatum, lanolin, or parabens.
CLINICAL PHARMACOLOGY:
Topical corticosteroids share anti-inflammatory, anti-pruritic and vasoconstrictive actions.
The mechanism of anti-inflammatory activity of the topical corticosteroids is unclear. Various laboratory methods, including vasoconstrictor assays, are used to compare and predict potencies and/or clinical efficacies of the topical corticosteroids. There is some evidence to suggest that a recognizable correlation exists between vasoconstrictor potency and therapeutic efficacy in man.
Pharmacokinetics. The extent of percutaneous absorption of topical corticosteroids is determined by many factors including the vehicle, the integrity of the epidermal barrier, and the use of occlusive dressings.
The topical corticosteroids can be absorbed from normal intact skin. Inflammation and/or other disease processes in the skin increase percutaneous abs...