Carteolol
Generic name: Carteolol hydrochloride
Dosage form: ophthalmic solution
Drug class:Ophthalmic glaucoma agents
Medically reviewed by Drugs.com. Last updated on Aug 1, 2021.
On This Page
Carteolol Description
Carteolol Hydrochloride Ophthalmic Solution USP, 1% is a nonselective beta-adrenoceptor blocking agent for ophthalmic use.
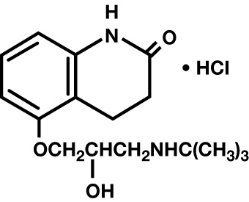
The chemical name for Carteolol hydrochloride is (±)-5-[3-[(1,1-dimethylethyl) amino]-2-hydroxypropoxy]-3,4-dihydro-2(1H)-quinolinone monohydrochloride. The structural formula is as follows:
C16H24N2O3•HCI Mol. Wt. 328.84
Each mL of sterile solution contains Active: Carteolol hydrochloride 10 mg (1%). Preservative: benzalkonium chloride 0.05 mg (0.005%). Inactives: sodium chloride, monobasic and dibasic sodium phosphate, sodium hydroxide and/or hydrochloric acid (to adjust pH to 6.0 - 8.0) and purified water.
Carteolol - Clinical Pharmacology
Carteolol is a nonselective beta-adrenergic blocking agent with associated intrinsic sympathomimetic activity and without significant membrane-stabilizing activity.
Carteolol Hydrochloride reduces normal and elevated intraocular pressure (IOP) whether or not accompanied by glaucoma. The exact mechanism of the ocular hypotensive effect of beta-blockers has not been definitely demonstrated.
In general, beta-adrenergic blockers reduce cardiac output in patients in good and poor cardiovascular health. In patients with severe impairment of myocardial function, beta-blockers may inhibit the sympathetic stimulation necessary to maintain adequate cardiac function. Beta-adrenergic blockers may also increase airway resistance in the bronchi and bronchioles due to unopposed parasympathetic activity.
Given topically twice daily in controlled domestic clinical trials ranging from 1.5 to 3 months, Carteolol Hydrochloride produced a median percent reduction of IOP 22% to 25%. No significant effects were noted on corneal sensitivity, tear secretion, or pupil size.