Dosage form: tablet, film coated, extended release
Drug class:Second generation cephalosporins
Medically reviewed by Drugs.com. Last updated on Jun 21, 2021.
On This Page
To reduce the development of drug-resistant bacteria and maintain the effectiveness of cefaclor extended-release tablets USP and other antibacterial drugs, cefaclor extended-release tablets USP should be used only to treat or prevent infections that are proven or strongly suspected to be caused by bacteria.
Cefaclor Extended Release Tablets Description
Cefaclor, USP, the active ingredient in cefaclor extended-release tablets USP, is a semisynthetic cephalosporin antibiotic for oral administration. Cefaclor, USP, is chemically designated as 3-chloro-7-D(2-phenylglycinamido)-3-cephem-4-carboxylic acid monohydrate. The cefaclor extended-release tablets formulation of cefaclor differs pharmacokinetically from the immediate-release formulation of cefaclor.
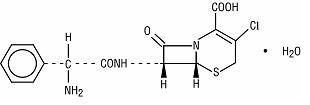
C15H14ClN3O



