CIS-PYRO
Generic name: sodium pyrophosphate and stannous chloride
Dosage form: injection
On This Page
CIS-PYRO Description
CIS-PYRO Kit for the Preparation of Technetium Tc 99m Pyrophosphate Injection is a multidose reaction vial which contains the sterile, non-pyrogenic, non-radioactive ingredients necessary to produce Technetium Tc 99m Pyrophosphate Injection for diagnostic use by intravenous injection.
Each 10 mL vial contains 12.0 mg of sodium pyrophosphate, 2.8 mg minimum stannous tin as stannous chloride dihydrate and 4.9 mg maximum total tin as stannous chloride dihydrate; pH is adjusted to 5.3-5.7 with hydrochloric acid prior to lyophilization. No bacteriostatic preservative is present. Sealed under nitrogen.
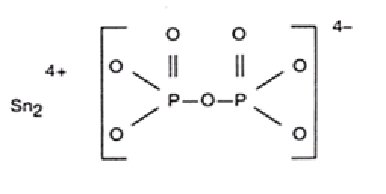
The chemical names are: (1) Diphosphoric acid, Ditin (2+) salt; (2) Ditin (2+) pyrophosphate (4‾). The structural formula is:
When a solution of sterile, non-pyrogenic, oxidant-free isotonic Sodium Pertechnetate Tc99m Injection U.S.P. is added to the vial, Technetium Tc99m Pyrophosphate Injection is formed for intravenous administration.
When a solution of sterile, non-pyrogenic, isotonic saline is added to the vial, it forms a blood pool imaging agent when Sodium Pertechnetate Tc 99m Injection is injected intravenously 30 minutes after the intravenous administration of the non-radioactive reconstituted CIS-PYRO. The precise structure of Technetium Tc 99m Pyrophosphate Injection is not known at this time.
Physical Characteristics
Technetium Tc 99m decays by isomeric transition with a physical half-life of 6.02 hours.¹ The principal photon that is useful for detection and imaging studies is listed in Table 1.
Table 1. Principal Radiation Emission Data| Radiation | Mean Percent Per DIsintegration | Mean Energy (keV) |
|
MEDICAL DEPARTMENTS
Cardiology
Pediatrics
Diabetes Care
Pre-natal Care
Ultrasound Echocardiogram
|



