Cysto-Conray II
Generic name:iothalamate meglumine
Dosage form: injection
Drug class:Ionic iodinated contrast media
Medically reviewed by Drugs.com. Last updated on Dec 1, 2020.
On This Page
Cysto-Conray II Description
Cysto-Conray II is a sterile aqueous solution intended for instillation as a diagnostic radiopaque medium. Cysto-Conray II contains 17.2% w/v iothalamate meglumine which is 1-Deoxy-1-(methylamino)-D-glucitol 5-acetamido-2,4,6-triiodo-N-methylisophthalamate (salt) and has the following structural formula:
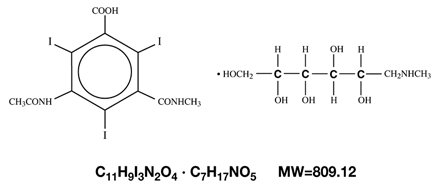
Each milliliter of Cysto-Conray II contains 172 mg of iothalamate meglumine, equivalent to 81 mg (8.1% w/v) of organically bound iodine, 0.110 mg edetate calcium disodium as a stabilizer and 0.115 mg of monobasic sodium phosphate as a buffer.
Cysto-Conray II is hypertonic under conditions of use and is supplied in containers from which the air has been displaced by nitrogen. The pH of Cysto-Conray II is
6.6 to 7.6.
Cysto-Conray II - Clinical Pharmacology
The most important characteristic of contrast media is the iodine content. The relatively high atomic weight of iodine contributes sufficient radiodensity for radiographic contrast.
Following instillation by sterile catheter, Cysto-Conray II provides for visualization of the lower urinary tract. Clinical literature reports indicate that routinely less than 1 percent of a retrograde urographic radiopaque is absorbed systemically, however, as much as 12 percent absorption was observed with pyelorenal back flow and may produce iodine medicated thyrotropic effects described under PRECAUTIONS.
Indications and Usage for Cysto-Conray II
Cysto-Conray II is indicated for use in retrograde cystography and cystourethrography.
<..


